
EXHIBIT 99.1

Corporate Presentation Updated February 2014

2 There are statements in this presentation that are not historical facts . These “forward - looking statements” can be identified by use of terminology such as “anticipate,” “believe,” “estimate,” “expect,” “hope,” “intend,” “may,” “plan,” “positioned,” “project,” “propose,” “should,” “strategy,” “will,” or any similar expressions . You should be aware that these forward - looking statements are subject to risks and uncertainties that are beyond our control . For a discussion of these risks, you should read the information that we have filed with the Securities and Exchange Commission, including our Current Report on Form 8 - K that was filed on February 7 , 2014 , especially the risks discussed under the section entitled “Risk Factors” in that Current Report and similar sections that will be provided in other filed reports . Although we believe that our assumptions underlying such forward - looking statements are reasonable, we do not guarantee our future performance, and our actual results may differ materially from those contemplated by these forward - looking statements . Our assumptions used for the purposes of the forward - looking statements specified in the following information represent estimates of future events and are subject to uncertainty as to possible changes in economic, legislative, industry, and other circumstances, including the development, acceptance and sales of our products and our ability to raise additional funding sufficient to implement our strategy . As a result, the identification and interpretation of data and other information and their use in developing and selecting assumptions from and among reasonable alternatives require the exercise of judgment . In light of these numerous risks and uncertainties, we cannot provide any assurance that the results and events contemplated by our forward - looking statements contained in this presentation will in fact transpire . You are cautioned to not place undue reliance on these forward - looking statements, which speak only as of their dates . We do not undertake any obligation to update or revise any forward - looking statements . Forward - Looking Statement Disclaimer

3 Cardax: Focusing on the Source of Inflammation TM Cardax is developing astaxanthin therapies for large unmet medical needs where inflammation and oxidative stress play key causative roles. CARDAX IN BRIEF ▪ Cardax is focusing first on developing products utilizing astaxanthin, a naturally occurring compound demonstrated to reduce inflammation without the harmful side effects of current anti - inflammatory treatments (e.g. steroids and NSAIDs). ▪ In 2006, Cardax and BASF entered into a Joint Development and Supply Agreement (“JDSA”) related to the development of a proprietary and scalable process to cost - effectively manufacture a competitively differentiated, pharmaceutical - grade, “nature - identical” astaxanthin with a defined molecular structure ("ASTX - 1"). ▪ BASF has exclusively licensed rights from Cardax to develop and commercialize ASTX - 1 in nutraceutical products (“BASF Astaxanthin Products”), and will pay Cardax royalties on future sales of BASF Astaxanthin Products. Human clinical trials are not required for nutraceutical product approvals. ▪ Cardax retains the exclusive rights to use ASTX - 1 in pharmaceutical products ("Cardax Astaxanthin") and plans to pursue pharmaceutical development, targeting conditions where inflammation and oxidative stress are strongly implicated.

4 About Cardax Cardax oral anti - inflammatory products address underserved multi - billion dollar chronic disease markets. CORPORATE HIGHLIGHTS ▪ Cardax believes that the manufacturing advantages brought about by the JDSA will provide an efficient, scalable, and economical path to mass markets not available to low - volume agricultural astaxanthin producers. ▪ Major pharmaceutical opportunities may be pursued; Cardax plans to conduct human clinical trials in order to support nutraceutical marketing efforts as well as over - the - counter and/or prescription drug approval. ▪ Astaxanthin products are supported by extensive research including 1,000+ peer reviewed papers and 40+ human clinical trials. ▪ Cardax patents protect compositions of matter, pharmaceutical compositions, and pharmaceutical uses. ▪ Cardax management has strong operational and technical expertise in the field of astaxanthin manufacturing and science.

5 Inflammation and Disease Cardax’s pharmaceutical development program will target conditions where inflammation and oxidative stress are strongly implicated. CONDITIONS TARGETED ▪ Osteoarthritis ▪ Cognitive Decline ▪ Metabolic Syndrome ▪ Liver Disease (NASH, ASH) ▪ Cardiovascular Disease (Atherosclerosis, Stroke) ▪ Neurological Disease (Alzheimer’s, Parkinson’s) ▪ Ophthalmic Disease (Dry, Wet AMD, Stargardt ) ▪ Prostate Disease ( Prostatitis , Prostate Cancer) Time Cover Story, February 2004

6 Inflammation and Disease Cont. Inflammation is the body's natural response to injury, irritants, and pathogens – chronic inflammation may occur when the immune system does not function properly and increases disease risk. CHRONIC INFLAMMATION ▪ Excess oxidative stress inside cells at the mitochondrial level is caused by many factors including aging, obesity, and smoking. ▪ Excess oxidative stress leads to the chronic and dysfunctional activation of cell - signaling pathways that produce inflammation. ▪ Chronic inflammation occurs in various tissues including the heart, lung, brain, liver, prostate, eye, and joints. ▪ Inflammation manifests itself in a wide variety of disease states. ▪ The typical approach to anti - inflammatory therapy is to treat symptoms with NSAIDs, such as aspirin or COX - 2 inhibitors, or to suppress the immune response with immune suppressive agents or steroids.

7 Astaxanthin Fights Inflammation at Its Source Astaxanthin reduces the oxidative stress that initiates and perpetuates the chronic and pathological activation of a number of inflammatory pathways. MECHANISM OF ACTION ▪ Astaxanthin spans plasma, mitochondrial, and nuclear membranes of the targeted tissue, stabilizing these membranes and reducing or preventing lipid peroxidation , including the oxidation of LDL. ▪ Astaxanthin’s localization as well as its anti - oxidant and related anti - inflammatory effects reduce the oxidative stress that initiates and/or perpetuates the chronic and pathological activation of a number of inflammatory pathways, including NF - KB, JNK, and others.

8 Astaxanthin in Nature Astaxanthin is a naturally occurring marine compound that was found to improve animal health and vitality, prompting further scientific study. ROLE OF ASTAXANTHIN IN THE MARINE FOOD CHAIN 1 3 2 Astaxanthin is synthesized by microalgae; astaxanthin is believed to protect the algae from environmental stress. Krill are small crustaceans that feed on the microalgae. Krill are eaten by higher order marine animals including whales, seals, and salmon.

9 Astaxanthin Scientific Research 1,000+ peer reviewed papers featuring astaxanthin have been published in leading scientific journals. RESEARCH SUPPORTS USE ▪ More than 40 human clinical trials support the safety and efficacy of astaxanthin. ▪ More than 50 peer reviewed papers published by Cardax team members. ▪ Ten papers have been published in The American Journal of Cardiology .
Astaxanthin Scientific Research Cont. Astaxanthin and Related Esters have demonstrated efficacy in models of inflammatory - mediated disease. DEMONSTRATED EFFICACY IN SCIENTIFIC RESEARCH ▪ Reduction of TNF - α levels equivalent to a steroid. ▪ Reduction of liver enzymes and liver histological damage. ▪ Reduction of cholesterol levels. ▪ Reduction of elevated triglycerides. ▪ Decrease of atheroma formation. ▪ Reduction of oxidized - LDL levels. ▪ Reduction in blood clot formation with no increase in bleeding. ▪ Decrease in myocardial tissue damage following experimentally - induced myocardial infarction. Source: See CardaxPharma.com for select astaxanthin research.
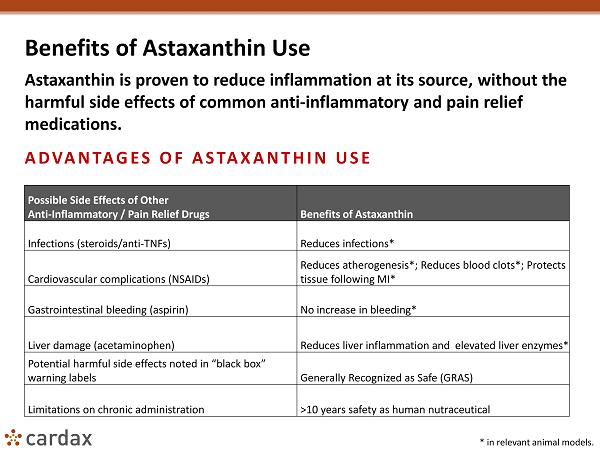
Benefits of Astaxanthin Use Astaxanthin is proven to reduce inflammation at its source, without the harmful side effects of common anti - inflammatory and pain relief medications. ADVANTAGES OF ASTAXANTHIN USE * in relevant animal models. Possible Side Effects of Other Anti - Inflammatory / Pain Relief Drugs Benefits of Astaxanthin Infections (steroids/anti - TNFs) Reduces infections* Cardiovascular complications (NSAIDs) Reduces atherogenesis *; Reduces blood clots*; Protects tissue following MI * Gastrointestinal bleeding (aspirin) No increase in bleeding* Liver damage (acetaminophen) Reduces liver inflammation and elevated liver enzymes* Potential harmful side effects noted in “black box” warning labels Generally Recognized as Safe (GRAS) Limitations on chronic administration >10 years safety as human nutraceutical
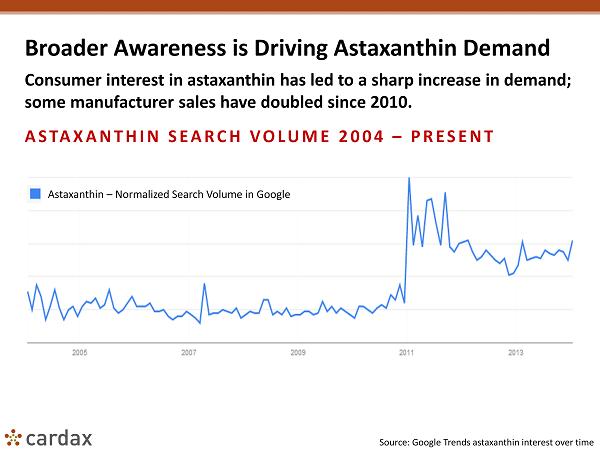
Broader Awareness is Driving Astaxanthin Demand Consumer interest in astaxanthin has led to a sharp increase in demand; some manufacturer sales have doubled since 2010. ASTAXANTHIN SEARCH VOLUME 2004 – PRESENT Source: Google Trends astaxanthin interest over time Astaxanthin – Normalized Search Volume in Google
Recent Coverage of Astaxanthin in Mainstream Media SELECT MEDIA COVERAGE All registered trademarks, logos, images, and content are property of their respective owners; there is no endorsement of Car dax or any its products or technologies by any such owner.

14 Agricultural Astaxanthin Currently, astaxanthin for nutraceutical use is primarily derived from low volume agricultural ( microalgal ) sources; agricultural producers face significant challenges to meet growing global demand. CERTAIN ISSUES FACED BY AGRICULTURAL SUPPLIERS The astaxanthin nutraceutical market is currently served by a small number of suppliers that grow microalgae ( Haematococcus pluvialis ) using agricultural methods. ▪ Current capacity is limited. ▪ Mass market capacity would require significant if not overwhelming investment. ▪ Agricultural astaxanthin yields are variable and can be impacted by weather fluctuations and disease. ▪ Agricultural astaxanthin production requires dry, tropical conditions or infrastructure - intensive closed systems. ▪ Higher dosing for pharmaceutical applications may be impractical given low astaxanthin content (5% to 15% by volume).

15 “Nature - Identical” Synthetic Astaxanthin The Cardax - BASF JDSA will enable an efficient, scalable, and economical path to mass markets not currently available to low - volume agricultural producers. NOVEL MANUFACTURING APPROACH ▪ A proprietary and scalable manufacturing process for the efficient and economical production of “nature - identical” synthetic astaxanthin in high volumes for nutraceutical and pharmaceutical use. ▪ Cardax believes “nature - identical” synthetic astaxanthin products with high - purity, reliable large - volume supply, and batch - to - batch consistency will increase astaxanthin market acceptance among consumers and suppliers. ▪ Few mass market nutraceuticals or pharmaceuticals use agricultural production methods – most use synthetic manufacturing methods.

16 “Nature - Identical” Synthetic Astaxanthin Cont. Production of “nature - identical” synthetic astaxanthin has multiple advantages over agricultural astaxanthin. SYNTHETIC VS. AGRICULTURAL PRODUCTION ▪ Synthetic production methods can be scaled to accommodate growing global demand with consistent supply and economical production costs. ▪ Synthetic production methods are clean, controlled, and efficient. ▪ Synthetic astaxanthin can achieve pharmaceutical - grade purity.

17 Cardax Astaxanthin: Pharmaceutical Development Cardax intends to pursue the clinical development of astaxanthin as an over - the - counter drug and/or prescription drug. CLINICAL DEVELOPMENT PROGRAM ▪ Cardax plans to conduct three to five low - risk, low - cost, clinical trials after obtaining additional financing or through strategic alliances. ▪ The clinical trials should help establish the safety and efficacy of Cardax Astaxanthin (clinical trials are not required for marketing astaxanthin as a nutraceutical). ▪ The clinical trials should increase consumer awareness of astaxanthin and may promote nutraceutical sales of BASF Astaxanthin. ▪ Cardax will seek U.S. FDA approval for: ▪ Over - the - counter drug approval based on a nutraceutical dose. ▪ Prescription drug approval for higher doses (e.g. 5x or 10x approved nutraceutical dose).
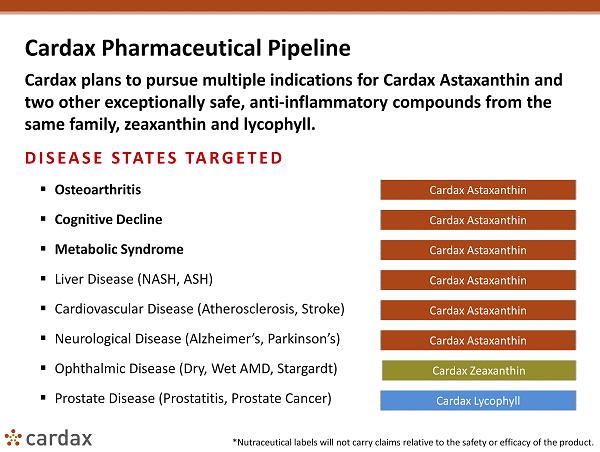
Cardax Pharmaceutical Pipeline Cardax plans to pursue multiple indications for Cardax Astaxanthin and two other exceptionally safe, anti - inflammatory compounds from the same family, zeaxanthin and lycophyll . DISEASE STATES TARGETED ▪ Osteoarthritis ▪ Cognitive Decline ▪ Metabolic Syndrome ▪ Liver Disease (NASH, ASH) ▪ Cardiovascular Disease (Atherosclerosis, Stroke) ▪ Neurological Disease (Alzheimer’s, Parkinson’s) ▪ Ophthalmic Disease (Dry, Wet AMD, Stargardt ) ▪ Prostate Disease ( Prostatitis , Prostate Cancer) Cardax Zeaxanthin Cardax Lycophyll Cardax Astaxanthin Cardax Astaxanthin Cardax Astaxanthin Cardax Astaxanthin Cardax Astaxanthin Cardax Astaxanthin *Nutraceutical labels will not carry claims relative to the safety or efficacy of the product.

19 Astaxanthin for Osteoarthritis Cardax Astaxanthin is well positioned to meet the needs of patients suffering from osteoarthritis. OSTEOARTHRITIS OPPORTUNITY ▪ Current treatments for osteoarthritis include NSAIDs (Celebrex®, Aleve®, etc.), steroids (Prednisolone, etc.), and the nutraceutical glucosamine/chondroitin. ▪ Astaxanthin is far safer than NSAIDs and steroids and more efficacious than glucosamine/chondroitin. ▪ Differentiation between nutraceutical astaxanthin and Cardax Astaxanthin can be achieved through “disease” labeling and/or dose. ▪ The Osteoarthritis market is so large that similar pricing per mg to nutraceutical astaxanthin still allows significant potential for pharmaceutical Cardax Astaxanthin. ▪ Astaxanthin is a powerful anti - inflammatory with demonstrated efficacy: ▪ TNF - α reduction confirmed in humans. ▪ Beneficial modulation of COX - 2, PGE2, IL - 1, iNOS, NO, and NF - κ B. ▪ Direct scavenger of superoxide anion, potentially helping to reduce pain.

Astaxanthin for Osteoarthritis Cont. The Centers for Disease Control and Prevention report that the amount of direct medical expenditures in the U.S. for arthritis and other rheumatic conditions was $80.8B in 2003. OSTEOARTHRITIS MARKET OVERVIEW ▪ According to the Centers for Disease Control and Prevention, one in every five American adults have doctor - diagnosed arthritis. As the country's population ages, it is estimated that this number will increase to at least 67% by 2030. ▪ Aggregate U.S. sales of the top three injected TNF - α inhibitors totaled more than $12B in 2012. ▪ Cardax estimates that there are more than 150 million people in developed nations that suffer from osteoarthritis who have the financial ability to pay for Cardax Astaxanthin treatments. Sources: CDC.gov; Drugs.com.

21 Astaxanthin for Cognitive Decline A human clinical trial supports the potential for Cardax Astaxanthin to improve cognitive function in an elderly population afflicted with age - related forgetfulness. COGNITIVE DECLINE OPPORTUNITY ▪ Potential treatment for an expanding population with few options to help slow progression or delay onset of these diseases. ▪ While the underlying cause of cognitive decline still remains to be fully elucidated, many studies support the important pathophysiological role of oxidative stress and inflammation in both Alzheimer’s disease and Parkinson’s disease. ▪ According to the CDC, the number of U.S. adults aged 65 or older will more than double by 2030. ▪ As the percentage of elderly in the population continues to increase, the prevalence of diseases resulting in cognitive decline is expected to increase.

Astaxanthin for Metabolic Syndrome Cardax Astaxanthin could lessen the majority of physiological measures defining metabolic syndrome, and thereby decrease the risk of ensuing cardiovascular disease, diabetes, and liver disease. METABOLIC SYNDROME OPPORTUNITY ▪ Metabolic syndrome is a combination of medical disorders, including elevated triglycerides, lower HDL - C, higher LDL - C, elevated blood pressure, and fasting blood pressure, that together increase the risk of developing cardiovascular disease, diabetes, and liver disease. ▪ Astaxanthin has been shown to significantly lower triglycerides and LDL - C and increase HDL - C levels in humans. Similarly, in animal models of disease, astaxanthin administration significantly decreased blood pressure, increased HDL - C levels, lowered triglycerides, and decreased fasting glucose levels. ▪ The global metabolic syndrome market was estimated at US$97.7B in 2008.* *Source: Harvard University Office of Technology Development

23 Intellectual Property Cardax has a robust intellectual property portfolio that offers a valuable competitive advantage. PATENT PORTFOLIO ▪ Cardax patents protect compositions of matter, pharmaceutical compositions, and pharmaceutical uses of its Astaxanthin and related products in certain disease areas. ▪ 20 issued patents, including 13 in the U.S. and seven in China, India, Japan, and Hong Kong. These patents will expire between 2023 to 2028, subject to patent extensions. ▪ Cardax has one patent application pending in the U.S. and five patent applications pending in Europe, Canada, and Brazil, relating to its technology. ▪ Cardax maintains rights to a certain U.S. patent issued to Brigham and Women’s Hospital. The patent includes technology related to the pharmaceutical use of astaxanthin or other specified carotenoids for the amelioration of a major vascular event such as myocardial infarction, stroke, coronary revascularization, and cardiovascular death.

24 Senior Management Team DAVID G. WATUMULL, PRESIDENT & CEO, DIRECTOR More than twenty years industry experience as a biotechnology executive, analyst, and investment banker. Co - founder of Cardax and co - inventor of Cardax’s technology. Conceived and led development of first astaxanthin dietary supplement. While CEO of Hawaii Biotech, built a vaccine development program that subsequently resulted in a successful human proof of concept study and sale of related technology to Merck. GILBERT RISHTON, PH.D., CHIEF SCIENCE OFFICER Former drug discovery and development leader at Amgen. Built Amgen's Small Molecule Drug Discovery Group, managed chemistry for preclinical and clinical development of Sensipar, and led medicinal chemistry for Amgen's Secretase Team. Founder and Director of the Channel Islands Alzheimer's Institute. JOHN RUSSELL, CHIEF FINANCIAL OFFICER 19 years of accounting, finance, operations, and SEC reporting experience in biopharmaceutical and high - tech industries. From 2010 to the present, he has served as Chief Financial Officer for various privately - held start - up companies. Licensed CPA in Hawaii and has a B.A. in Economics/Accounting from Claremont McKenna College.

25 Senior Management Team Cont. TIMOTHY KING, PH.D., VP RESEARCH Former Staff Scientist at the Fred Hutchinson Cancer Research Center (FHCRC) in Seattle, Washington, Dr. King has over 22 years of combined academic and private sector scientific research experience including utilizing cell culture and animal model systems to address a wide range of topics including cardiovascular disease, liver disease, thrombosis, and cancer. DAVID M. WATUMULL, VP OPERATIONS Over ten years experience in biotechnology with Hawaii Biotech and Cardax Pharmaceuticals. Has day - to - day responsibility for the Company’s operations as well as relations with partners and contractors for the production and testing of Cardax compounds. Manages the Cardax intellectual property portfolio.

26 Board of Directors NICHOLAS MITSAKOS, EXECUTIVE CHAIRMAN Chairman and CEO of Arcadia Holdings, Inc., focusing on private equity investments globally. Arcadia’s financial partners include Trust Company of the West, a $150B investment company, and Canyon Partners, a $20B hedge fund. Mitsakos is also a senior advisor to Sardis Capital, a London - based merchant bank; and Franklin Templeton China, in Shanghai. He is an investor in over forty companies. FRANK HERRINGER, DIRECTOR Chairman of Transamerica since 1995, and served as CEO of Transamerica from 1991 to 1999, and President from 1986 to 1999. Herringer was elected Chairman of the Board of the combined AEGON USA and Transamerica Corporation in July 1999. Herringer continues to serve on the board of Amgen. DAVID G. WATUMULL, PRESIDENT & CEO, DIRECTOR More than twenty years industry experience as a biotechnology executive, analyst, and investment banker. Co - founder of Cardax and co - inventor of Cardax’s technology. Conceived and led development of first astaxanthin dietary supplement. While CEO of Hawaii Biotech, built a vaccine development program that subsequently resulted in a successful human proof of concept study and sale of related technology to Merck.

Selected Astaxanthin Clinical Trials
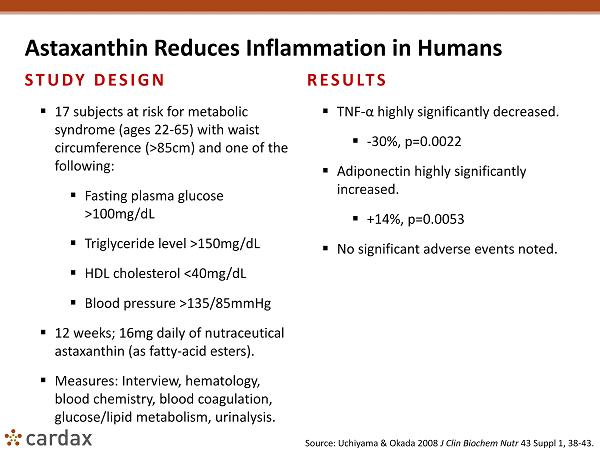
Astaxanthin Reduces Inflammation in Humans STUDY DESIGN ▪ 17 subjects at risk for metabolic syndrome (ages 22 - 65) with waist circumference (>85cm) and one of the following: ▪ Fasting plasma glucose >100mg/dL ▪ Triglyceride level >150mg/dL ▪ HDL cholesterol <40mg/dL ▪ Blood pressure >135/85mmHg ▪ 12 weeks; 16mg daily of nutraceutical astaxanthin (as fatty - acid esters). ▪ Measures: Interview, hematology, blood chemistry, blood coagulation, glucose/lipid metabolism, urinalysis. RESULTS ▪ TNF - α highly significantly decreased. ▪ - 30%, p=0.0022 ▪ Adiponectin highly significantly increased. ▪ +14%, p=0.0053 ▪ No significant adverse events noted. Source: Uchiyama & Okada 2008 J Clin Biochem Nutr 43 Suppl 1, 38 - 43.
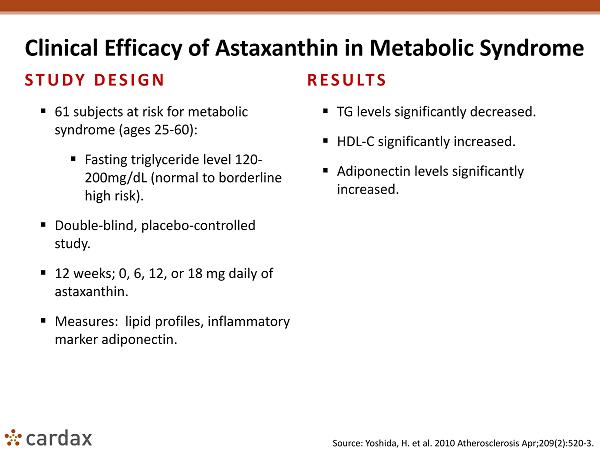
Clinical Efficacy of Astaxanthin in Metabolic Syndrome STUDY DESIGN ▪ 61 subjects at risk for metabolic syndrome (ages 25 - 60): ▪ Fasting triglyceride level 120 - 200mg/ dL (normal to borderline high risk). ▪ Double - blind, placebo - controlled study. ▪ 12 weeks; 0, 6, 12, or 18 mg daily of astaxanthin. ▪ Measures: lipid profiles, inflammatory marker adiponectin . RESULTS ▪ TG levels significantly decreased. ▪ HDL - C significantly increased. ▪ Adiponectin levels significantly increased. Source: Yoshida, H. et al. 2010 Atherosclerosis Apr;209(2):520 - 3.
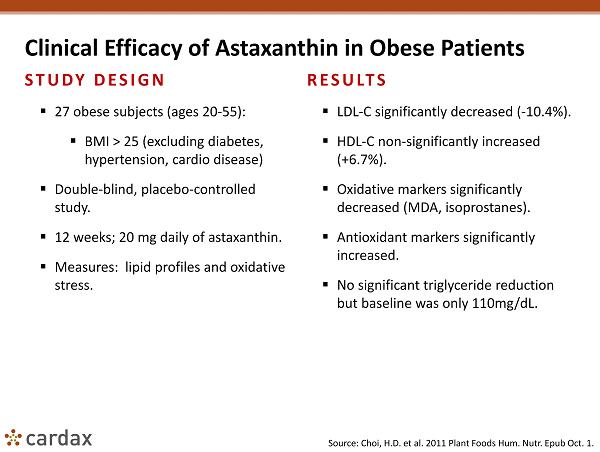
Clinical Efficacy of Astaxanthin in Obese Patients STUDY DESIGN ▪ 27 obese subjects (ages 20 - 55): ▪ BMI > 25 (excluding diabetes, hypertension, cardio disease) ▪ Double - blind, placebo - controlled study. ▪ 12 weeks; 20 mg daily of astaxanthin. ▪ Measures: lipid profiles and oxidative stress. RESULTS ▪ LDL - C significantly decreased ( - 10.4%). ▪ HDL - C non - significantly increased (+6.7%). ▪ Oxidative markers significantly decreased (MDA, isoprostanes ). ▪ Antioxidant markers significantly increased. ▪ No significant triglyceride reduction but baseline was only 110mg/ dL . Source: Choi , H.D. et al. 2011 Plant Foods Hum. Nutr . Epub Oct. 1.

31 The information contained in this Corporate Presentation (this “Presentation”) summarizes certain information about Cardax, Inc . (the “Company”) and its subsidiary Cardax Pharma , Inc . and is qualified in its entirety by, and should be considered in conjunction with, the information (the “Company Public Disclosures”) that is filed by the Company from time to time with the Securities and Exchange Commission in accordance with the Securities and Exchange Act of 1934 , as amended . The Company Public Disclosures include filings or reports that include a summary of certain risks and uncertainties or “Risk Factors” . Because this Presentation does not include all of the material information, you should carefully review the Company Public Disclosures . This Presentation and the other information provided by the Company through its website or that may be accessed through the Company’s website is not part of the Company Public Disclosures unless it is filed with the Securities and Exchange Commission . This Presentation includes market and industry data and forecasts that we obtained from industry publications and surveys and other sources . Industry publications and surveys and forecasts generally state that the information contained therein has been obtained from sources believed to be reliable, but there can be no assurance as to the accuracy or completeness of such included information . We have not independently verified any of the data from third - party sources nor have we ascertained the underlying economic assumptions relied upon therein . Statements as to market position or consumer demand are based on data currently available to us . While we are not aware of any misstatements regarding our industry data presented herein, our estimates involve risks and uncertainties and are subject to change based on various factors . All registered trademarks, service marks, logos, images, and content are property of their respective owners and there is no endorsement of Cardax or any its products or technologies by any such owner . No offer of securities is made, or solicited, by the Company through this Presentation . The Company is not providing any legal, business, tax or other advice in this Presentation . No Governmental Agency or other authority has approved any aspect of the Company, its operations or its products and no Governmental Agency or other authority has approved or will approve the offering of any securities issued by the Company . Any representation to the contrary is unlawful . Certain Disclosures

Cardax, Inc. 2800 Woodlawn Drive, Suite 129 Honolulu, Hawaii 96822 (808) 457 - 1400