UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
FORM 10-K
[X] Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
For the fiscal year ended December 31, 2019
[ ] Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
For the transition period from ___________ to ___________
Commission File Number 333-181719
CARDAX, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 45-4484428 | |
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
2800 Woodlawn Drive, Suite 129 Honolulu, Hawaii |
96822 | |
| (Address of principal executive offices) | (Zip code) |
(808) 457-1400
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: None
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| N/A | N/A | N/A |
Note: The registrant’s common stock,
par value $0.001, is quoted under the symbol “CDXI” on the OTCQB
but is not registered under Section 12(b) of the Act.
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes [ ] No [X]
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act.
Yes [ ] No [X]
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes [X] No [ ]
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes [X] No [ ]
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [X]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company in Rule 12b-2 of the Exchange Act.
| Large accelerated filer [ ] | Accelerated filer [ ] |
| Non-accelerated filer [ ] (Do not check if a smaller reporting company) | Smaller reporting company [X] |
| Emerging growth company [ ] |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. [ ]
Indicate by check whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes [ ] No [X]
As of June 30, 2019, the last business day of the registrant’s most recently completed second fiscal quarter, there were 683,731 shares of common stock, par value $0.001 per share (“common stock”), outstanding, and 643,953 shares were held by non-affiliates. As of such date, the aggregate market value of voting and non-voting common equity held by non-affiliates was $ $16,098,825.
As of March 30, 2020, there were 758,973 shares of common stock of the registrant outstanding.
TABLE OF CONTENTS
| 2 |
Explanatory Note
Unless otherwise noted, references in this Annual Report on Form 10-K to “Cardax,” the “Company,” “we,” “our,” or “us” means Cardax, Inc., the registrant, and, unless the context otherwise requires, together with its wholly-owned subsidiary, Cardax Pharma, Inc., a Delaware corporation (“Pharma”), and Pharma’s predecessor, Cardax Pharmaceuticals, Inc., a Delaware corporation (“Holdings”), which merged with and into Cardax, Inc. on December 30, 2015.
Unless otherwise noted, references in this Annual Report on Form 10-K to our “product” or “products” includes our dietary supplements, pharmaceutical candidates, and any of our other current or future products, product candidates, and technologies, to the extent applicable.
Special Note Regarding Forward-Looking Statements
There are statements in this annual report that are not historical facts. These “forward-looking statements” can be identified by use of terminology such as “anticipate,” “believe,” “estimate,” “expect,” “hope,” “intend,” “may,” “plan,” “positioned,” “project,” “propose,” “should,” “strategy,” “will,” or any similar expressions. You should be aware that these forward-looking statements are subject to risks and uncertainties that are beyond our control. For a discussion of these risks, you should read this entire annual report carefully, especially the risks discussed under the section entitled “Risk Factors.” Although we believe that our assumptions underlying such forward-looking statements are reasonable, we do not guarantee our future performance, and our actual results may differ materially from those contemplated by these forward-looking statements. Our assumptions used for the purposes of the forward-looking statements specified in the following information represent estimates of future events and are subject to uncertainty as to possible changes in economic, legislative, industry, and other circumstances, including the development, acceptance, and sales of our products, the continued availability of our exclusive “brick and mortar” sales channel for our commercial product, and our ability to raise additional funding sufficient to implement our strategy. As a result, the identification and interpretation of data and other information and their use in developing and selecting assumptions from and among reasonable alternatives require the exercise of judgment. In light of these numerous risks and uncertainties, we cannot provide any assurance that the results and events contemplated by our forward-looking statements contained in this annual report will in fact transpire. These forward-looking statements are not guarantees of future performance. You are cautioned to not place undue reliance on these forward-looking statements, which speak only as of their dates. We do not undertake any obligation to update or revise any forward-looking statements, except as required by law.
Cautionary Note Regarding Industry Data
Unless otherwise indicated, information contained in this annual report concerning our company, our business, the services we provide and intend to provide, our industry and our general expectations concerning our industry are based on management estimates. Such estimates are derived from publicly available information released by third party sources, as well as data from our internal research, and reflect assumptions made by us based on such data and our knowledge of the industry, which we believe to be reasonable.
Corporate Information
We are a development stage biopharmaceutical company engaged in the development and commercialization of pharmaceuticals and dietary supplements. We are a smaller reporting company as defined by applicable federal securities regulations. We are a Delaware corporation.
Our executive offices are located at 2800 Woodlawn Drive, Suite 129, Honolulu, Hawaii 96822; our telephone number is (808) 457-1400. Our website is located at https://www.cardaxpharma.com. The information on our website is not part of this annual report.
Reverse Stock Split
On January 15, 2020, we effected a 200-for-1 reverse stock split (the “Reverse Stock Split”) of our issued and outstanding shares of common stock. The Reverse Stock Split did not change the number of shares of our common stock authorized for issuance, the par value of our common stock, or any other terms of our common stock. No fractional shares were issued in the Reverse Stock Split and any remaining share fractions were rounded up to the next whole share. Under the terms and conditions of outstanding options, warrants, and other convertible securities, the number of underlying shares of our common stock and the exercise prices or conversion prices thereof were proportionately adjusted for the Reverse Stock Split.
Proposed Public Offering
We filed a registration statement on Form S-1 on August 14, 2019, as amended September 27, 2019 and November 22, 2019, for a proposed $15 million public offering of our common stock and warrants and the listing of our common stock and such warrants on the Nasdaq Capital Market (the “Proposed Public Offering”). We intend to use the proceeds from the Proposed Public Offering primarily to fund pharmaceutical development and our operations. Notwithstanding the uncertain market conditions related to coronavirus disease 2019 (“COVID-19”), we plan to continue to take actions to consummate the Proposed Public Offering. We cannot give any assurance that the Proposed Public Offering will be consummated.
| 3 |
Our Business
We are a development stage biopharmaceutical company focused primarily on the development of pharmaceuticals to safely address one of the major underlying causes of many chronic diseases – inflammation – including cardiovascular disease, metabolic disease, liver disease, arthritis, and aging. We also have a commercial business unit that markets dietary supplements for inflammatory health. We believe we are well positioned for growth through the utilization of astaxanthin and zeaxanthin for chronic pharmaceutical applications by safely reducing chronic inflammation at the cellular and mitochondrial level – without inhibiting normal function. Similar mechanisms also support the use of our dietary supplement for inflammatory health.
We believe that our pharmaceutical product candidates and our dietary supplements have competitive advantages, primarily relating to a unique combination of the following benefits:
| ● | An excellent safety profile that supports chronic use | |
| ● | Broad anti-inflammatory activity and pleiotropic effects with potential application to several chronic diseases as pharmaceuticals and various areas of health as dietary supplements | |
| ● | Oral dosing convenience | |
| ● | Scalable manufacturing | |
| ● | Economical pricing |
Market Overview
There is broad acceptance in the scientific, medical, and financial communities that chronic inflammation is a significant factor in many chronic diseases, particularly cardiovascular disease. The double-blind, randomized, placebo-controlled CANTOS clinical trial (10,061 patients; Novartis, 2017) and REDUCE-IT clinical trial (8,179 patients; Amarin Corporation, 2018), both published in the New England Journal of Medicine, helped to catalyze and support this consensus. Commonly used anti-inflammatory drugs such as aspirin, ibuprofen, naproxen, COX-2 inhibitors, corticosteroids, and various biologics may reduce inflammation, but they have risks of significant side effects that limit their utility in chronic disease.
We believe that a safe anti-inflammatory is the solution. Our lead pharmaceutical candidate CDX-101, a proprietary prodrug of the naturally occurring marine molecule astaxanthin, may provide the needed combination of an excellent safety profile, anti-inflammatory activity, and economic pricing to become widely used for the prevention and treatment of chronic diseases driven by inflammation.
We are pursuing an initial indication of severe hypertriglyceridemia (triglycerides ≥ 500 mg/dL) for CDX-101. Severe hypertriglyceridemia is associated with chronic inflammation and patients with the disorder have increased cardiovascular disease risk and incidence of pancreatitis. We believe the clinical pathway to FDA drug approval for severe hypertriglyceridemia, which relies on biomarker endpoints (i.e., measuring triglycerides in blood tests over a period of several months), will be more efficient than other potential indications that require clinical outcomes studies (e.g., evaluating heart attacks, strokes, and deaths over a period of several years), and is thus better suited as our initial indication for CDX-101.
An estimated 3.4 million Americans have severe hypertriglyceridemia according to peer-reviewed research published in the American Journal of Cardiology in 2011. Statins, fibrates, and prescription fish oils are all used to manage hypertriglyceridemia. 21% (42 million) of U.S. adults have mixed dyslipidemia (high levels of low-density lipoprotein “LDL” cholesterol with low levels of high-density lipoprotein “HDL” cholesterol and/or high levels of triglycerides), with nearly 6% (11.6 million people) having all three lipid abnormalities. Lovaza, Vascepa, and other prescription fish oils approved for severe hypertriglyceridemia are also used off-label in mixed dyslipidemia patients to reduce moderately elevated triglycerides and aggregate sales of these products for on and off-label use are estimated to be approaching $2 billion annually.
| 4 |
We believe CDX-101 will have several competitive advantages compared to prescription fish oils: (i) ease of administration: oral dosing of large fish oil capsules is problematic, whereas we expect CDX-101 tablets should be far smaller; (ii) scalability: prescription fish oil manufacturing is limited by the declining global fish supply, whereas we believe the synthetic production of CDX-101 is scalable; and (iii) safety: prescription fish oils have certain safety risks, whereas we believe that astaxanthin, the active moiety of CDX-101, has an excellent safety profile.
The REDUCE-IT clinical trial demonstrated that administration of Vascepa resulted in a significant reduction of major adverse cardiovascular events (“MACE”) in patients with mixed dyslipidemia on standard of care, specifically statins, and we believe is the primary basis of Amarin’s request to the FDA to expand Vascepa’s label. The reduction of triglycerides in the REDUCE-IT clinical trial was modest however, and the study’s authors concluded that Vascepa’s ability to reduce other markers of cardiovascular disease, including inflammation and oxidized LDL (as demonstrated in the MARINE and ANCHOR clinical trials), provided the pleiotropic effects that led to reduction of MACE in REDUCE-IT. In human proof-of-concept “pilot” studies conducted by third parties and animal models conducted by third parties and us, astaxanthin, the active moiety of CDX-101, has demonstrated similar pleiotropic effects, which are derived from its broad anti-inflammatory activity, but without the limitations of Vascepa or other prescription fish oils. As a result, we believe this market also presents a major opportunity as a potential second indication for CDX-101.
Beyond cardiovascular disease, we believe CDX-101 could be developed to address other chronic diseases driven by inflammation, including metabolic disease, liver disease, arthritis, and aging, each with potential annual sales exceeding a billion dollars.
We are also developing CDX-301, our zeaxanthin pharmaceutical candidate, for macular degeneration. Our target initial indication for CDX-301 is Stargardt disease, a juvenile form of macular degeneration and potential orphan drug indication. Zeaxanthin has a mechanism of action and excellent safety profile similar to astaxanthin, however, it accumulates in the human eye through uptake by a unique retinal receptor, providing protection against blue light, oxidative damage, and related inflammation that occurs in macular degeneration. Pre-clinical and clinical studies with zeaxanthin have demonstrated proof-of-concept for the treatment of macular disorders. Based on multiple academic and NIH sources, we believe there are no more than 42,000 persons in the United States with Stargardt disease, and therefore we believe a treatment for Stargardt disease may qualify for orphan drug designation. (By statute, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition that affects less than 200,000 persons in the United States.) If CDX-301 receives FDA orphan drug designation for Stargardt disease and obtains FDA drug approval, we expect CDX-301 may benefit from certain advantages as an orphan drug, including orphan drug exclusivity, which means the FDA may not approve any other application, including a full NDA, to market the same drug for the same indication for a period of seven years, except in limited circumstances. We also believe that age related macular degeneration, a larger market estimated to afflict more than three million people in the U.S. alone, presents a major opportunity as a potential second indication for CDX-301. We do not expect to use the proceeds of the Proposed Public Offering to pursue the development of CDX-301.
| 5 |
Astaxanthin
Astaxanthin Safety
Astaxanthin is a naturally occurring marine carotenoid found in salmon, microalgae, krill, lobster, and crab. Carotenoids are natural pigments that impart coloration and support animal health and vitality, especially in harsh marine environments. Astaxanthin is responsible for the characteristic red or pink color of salmon and shellfish. Salmon without astaxanthin are smaller, more susceptible to infection, have reproductive problems, and are not strong enough to swim upstream.
Astaxanthin is GRAS as a food substance according to FDA regulations and has undergone extensive toxicity testing by third parties and us with no clinically meaningful issues even at the extremely high doses summarized in the table below:
| Type of Study | Maximum Dosing | |
| Acute Toxicity | >8,000 mg/kg (mouse, rat), 2,000 mg/kg (non-human primates) | |
| Sub-Chronic Toxicity | 1,240 mg/kg (rat), 160 mg/kg (dog) | |
| 1 Year Chronic Toxicity/Carcinogenicity | 1,000 mg/kg (rat), 1,400 mg/kg (mouse), 200 mg/kg (dog) | |
| 2 Year Carcinogenicity | 1,000 mg/kg (rat) | |
| Genotoxicity/Mutagenicity | 2,000 mg/kg (mouse) | |
| Teratogenicity | 1,000 mg/kg (rat), 400 mg/kg (rabbit) |
Commonly used anti-inflammatory drugs such as aspirin, ibuprofen, naproxen, COX-2 inhibitors, corticosteroids, and various biologics have risks of side effects including gastrointestinal bleeding, heart attacks, strokes, and severe infections. Prescription fish oil drugs, while safer than common anti-inflammatory drugs, also have risks of certain side effects. Lovaza and other DHA, EPA combination fish oil drugs, have risks of side effects including back pain, eructation, dysgeusia, and increases in LDL cholesterol. Vascepa has risks of side effects including arthralgia, atrial fibrillation, and increased bleeding. Fenofibrates have risks of side effects including stomach pain, nausea, and back pain.
In contrast, astaxanthin has no known side effects of clinical significance. We believe astaxanthin’s excellent safety profile will be a key competitive advantage compared to other drugs targeting inflammation and lipids.
| 6 |
Astaxanthin Mechanism of Action
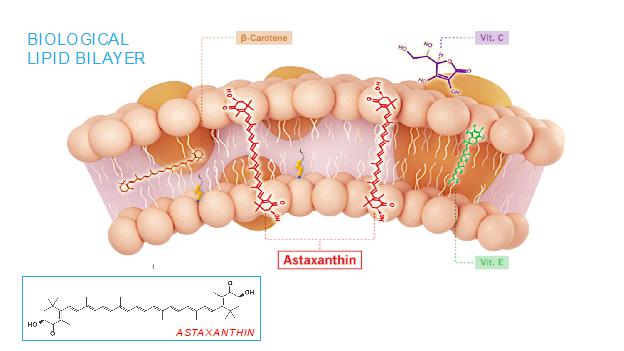
The mechanism of action of astaxanthin, the active moiety in CDX-101, is quite different than most drugs, and we believe is responsible for its excellent safety profile. Most drugs target single receptors or enzymes in complex pathways, which can lead to side effects with chronic use. Astaxanthin is distributed systemically, including to the liver and heart, where it localizes in cellular and mitochondrial membranes and reduces the oxidative stress that causes chronic inflammation, without affecting the normal function of inflammatory/metabolic signaling pathways. And unlike other antioxidants such as beta-carotene, Vitamin C, and Vitamin E, astaxanthin spans and stabilizes cellular and mitochondrial membranes (biological lipid bilayers) to function as an aqueous and lipid phase antioxidant without membrane disruption, as proven by X-ray diffraction studies:

As a result, astaxanthin demonstrates positive and quantifiable pleiotropic effects on many inflammatory cytokines and drug targets.
In human proof-of-concept “pilot” studies conducted by third parties, astaxanthin statistically significantly decreased inflammation and oxidative stress:
| ● | TNF-α decreased (-30%, p=0.0022) | |
| ● | CRP decreased (-20%, p<0.05; two studies) | |
| ● | Oxidative stress decreased (MDA, IsoP, SOD, TAC increased) |
In animal studies conducted by third parties, astaxanthin statistically significantly decreased inflammation and oxidative stress:
| ● | Inflammatory markers decreased in various model systems: |
| ○ | TNF-α, IL-1β, IL-6, CRP, NF-kB, PGE-2, iNOS, MCP-1, MPO, ERK, JNK, COX-2 | |
| ○ | TNF-α decreased equivalent to an equal dose of prednisolone |
| ● | Oxidative stress decreased in mitochondria |
| 7 |
Astaxanthin Research Results
There are more than 2,000 published peer reviewed papers related to astaxanthin, including more than 50 peer reviewed papers published by Cardax and its collaborators (referred to herein as “us”) and more than 50 “pilot” human clinical trials with astaxanthin supplements, more than 20 of which were randomized, double-blind, placebo-controlled human proof-of-concept studies. Highlights of astaxanthin’s pleiotropic effects, which were demonstrated in studies utilizing astaxanthin from natural and synthetic sources, include:
Astaxanthin and Cardiovascular Disease
In human proof-of-concept “pilot” studies conducted by third parties, astaxanthin statistically significantly decreased inflammation, triglycerides, LDL cholesterol, and blood pressure:
| ● | CRP decreased (-20%, p<0.05; two studies) | |
| ● | Triglycerides decreased (-25.8%, p<0.05) | |
| ● | LDL-C decreased (-10.4%, p<0.05) | |
| ● | HDL-C increased (+14.5%, p<0.01) | |
| ● | Apolipoprotein B decreased (-7.5%, p<0.01) | |
| ● | Adiponectin increased (+26%, p<0.01; +14%, p=0.0053; +30%, p=0.01; three studies) | |
| ● | Blood pressure decreased (systolic blood pressure -4.6%, p=0.021; diastolic blood pressure -6.9%, p<0.001; two studies) | |
| ● | Blood flow velocity increased (choroidal, p=0.018, blood transit time, p<0.01) |
In animal studies conducted by third parties and us, astaxanthin demonstrated statistically significant improvements in models of cardiovascular disease:
| ● | CRP and IL-6 decreased | |
| ● | Triglycerides decreased (plasma, hepatic) | |
| ● | Re-thrombosis decreased | |
| ● | Atherosclerosis decreased (aortic arch plaque) | |
| ● | Cholesterol decreased | |
| ● | Blood pressure decreased | |
| ● | Nitric oxide production increased |
Astaxanthin and Metabolic Disease
In human proof-of-concept “pilot” studies conducted by third parties, astaxanthin statistically significantly increased adiponectin and decreased TNF-α and oxidative stress:
| ● | Adiponectin increased (+26%, p<0.01; +14%, p=0.0053; +30%, p=0.01; three studies) | |
| ● | TNF-α decreased (-30%, p=0.0022) | |
| ● | Oxidative stress decreased (MDA, IsoP, SOD, TAC increased) |
In animal studies conducted by third parties, astaxanthin demonstrated statistically significant improvements in models of metabolic disease:
| ● | Fasting blood glucose levels decreased | |
| ● | Insulin levels & sensitivity (HOMA-IR, QUICK) increased | |
| ● | Insulin signaling (PI3K-AKT, IRS-1p) increased | |
| ● | Adiponectin levels increased | |
| ● | Insulin response and glucose tolerance (ipGTT) increased | |
| ● | GLUT-4 translocation increased | |
| ● | JNK, ERK-1 levels decreased | |
| ● | Nitric oxide production increased |
| 8 |
Astaxanthin and Liver Disease
In human proof-of-concept “pilot” studies conducted by third parties, astaxanthin statistically significantly decreased fat accumulation in biopsy-diagnosed NASH patients, decreased TNF-α, improved lipid profile parameters, and decreased oxidative stress:
| ● | NASH disease markers decreased in patients: |
| ○ | Steatosis: p<0.05 | |
| ○ | Nonalcoholic fatty liver disease (“NAFLD”) Activity Score (“NAS”): p<0.08 | |
| ○ | Lobular inflammation decreased: trend |
| ● | TNF-α decreased (-30%, p=0.0022) | |
| ● | Lipid profile parameters improved (LDL, HDL, ApoB, TG) | |
| ● | Oxidative stress decreased (MDA, IsoP, SOD, TAC increased) |
In animal studies conducted by third parties and us, astaxanthin statistically significantly decreased elevated liver enzymes, lipids, insulin resistance, steatosis, and fibrosis:
| ● | Elevated liver enzyme levels decreased | |
| ● | Steatosis decreased | |
| ● | Fibrosis and induced acute hepatitis decreased | |
| ● | Insulin levels & sensitivity (HOMA-IR, QUICK) increased | |
| ● | Insulin signaling (PI3K-AKT, IRS-1p) increased | |
| ● | Adiponectin levels increased |
Astaxanthin and Arthritis
In human proof-of-concept “pilot” non-arthritis studies conducted by third parties, astaxanthin statistically significantly decreased markers of inflammation of relevance to arthritis, including TNF-α and CRP:
| ● | TNF-α decreased (-30%, p=0.0022) | |
| ● | CRP decreased (-20%, p<0.05; two studies) | |
| ● | Adiponectin increased (+26%, p<0.01; +14%, p=0.0053; +30%, p=0.01; three studies) | |
| ● | Oxidative stress decreased (MDA, IsoP, SOD, TAC increased) |
In animal studies conducted by third parties, astaxanthin statistically significantly decreased inflammation, oxidative stress, and joint degeneration:
| ● | Inflammatory markers decreased in various model systems: |
| ○ | TNF-α, IL-1β, IL-6, CRP, NF-kB, PGE-2, iNOS, MCP-1, MPO, ERK, JNK, COX-2 | |
| ○ | TNF-α decreased equivalent to an equal dose of prednisolone |
| ● | Oxidative stress decreased in mitochondria | |
| ● | Cartilage degradation decreased (Mankin score) in surgically-induced model of OA (ACLT, rabbit) |
| 9 |
Astaxanthin and Aging
In human studies conducted by third parties, activation of the FOXO3 gene has been linked to decreased inflammation and aging.
| ● | Activation of anti-inflammatory, anti-aging gene FOXO3 promotes longevity in humans: |
| ○ | Replicated in >20 independent studies | |
| ○ | Confers CVD protective benefit (p=0.001) | |
| ○ | Decreases inflammation (CRP, trend; TNF-α, p=0.018) |
In animal studies conducted by third parties and us, astaxanthin statistically significantly decreased activated the FOXO3 gene and extended lifespan:
| ● | FOXO3 mRNA levels increased in mice by 90% (p=0.024) | |
| ● | Lifespan extended by up to 30% via FOXO3 ortholog DAF16 in roundworms |
Astaxanthin and Coronavirus Disease 2019 (COVID-19)
In response to the COVID-19 global pandemic, our scientific team examined published scientific literature related to COVID-19 disease pathology in order to determine if there was a potential role of astaxanthin in the treatment of COVID-19. On March 20, 2020, we released a scientific white paper authored by Timothy J. King, Ph.D., M.S., our Vice President, Research, which discussed the scientific rationale of boosting the immune system and reducing the extreme inflammatory response that may lead to severe respiratory complications in subjects with COVID-19 as described below. We are presently seeking strategic collaborations with appropriate academic, governmental, and/or commercial organizations to further develop astaxanthin for COVID-19.
Scientific Rationale: Immune Response, Inflammation, and COVID-19
COVID-19, the disease caused by the novel coronavirus (SARS-CoV-2), can induce an extreme immune response characterized by the overproduction of immune cells and the uncontrolled release of pro-inflammatory cytokines. Exceedingly high levels of the cytokines IL-1, IL-6, IL-8, TNF-α, and CRP result in overt inflammatory symptoms that include mild to severe respiratory disease, high fever, and cough. In progressed disease this “cytokine storm” will circulate throughout the body to trigger a surge of active immune cells into the lungs resulting in acute lung injury and acute respiratory distress syndrome (ARDS) and may be particularly severe in immune compromised subjects such as the elderly, diabetics, and those with cardiovascular disease. This association between infection and progression of disease with inflammation suggests a strategy that would partner anti-infective agents, such as anti-virals and vaccines, with anti-inflammatory agents. An anti-inflammatory agent would be expected to mitigate symptoms including fever, pain, and swelling. Furthermore, an anti-inflammatory regimen initiated at a relatively early stage in disease progression might be expected to stem the immune over-response and to slow or even prevent progression of symptoms leading to lung injury and ARDS. Importantly, an appropriate anti-inflammatory intervention should not result in abnormal immune suppression but should target healthy immune homeostasis. An anti-inflammatory treatment that resulted in the decrease of inflammatory cytokine signaling would seem a promising approach. Chinese researchers have identified IL-6 as a main driver of immune overreaction in COVID-19 patients and have already included elevated IL-6 levels as a biomarker of disease worsening. China’s National Health Commission has updated its treatment guidelines for COVID-19 to include Roche’s injected biologic, Actemra (tocilizumab), an inhibitor of the IL-6 receptor. Actemra, first approved by the U.S. FDA in 2010 for rheumatoid arthritis, can now be used in China to treat serious COVID-19 patients with lung damage.
The Potential Role of Astaxanthin
In pre-clinical and clinical studies, astaxanthin has demonstrated the ability to decrease levels of pro-inflammatory cytokines IL-1, IL-6, TNF-α, and CRP in multiple models of disease and in several patient populations. Furthermore, astaxanthin does not lead to abnormal immune suppression even at high doses and acts to restore healthy immune homeostasis. Astaxanthin has also demonstrated exceptional safety in multiple animal models, has been used extensively in humans for two decades as a dietary supplement, and is GRAS (Generally Recognized as Safe), according to FDA regulations. Coronaviruses have been shown to induce lung damage by increasing inflammatory signaling pathways and cytokine production leading to elevated immune cell infiltration and macrophagic polarization shifts (M2 to M1). Astaxanthin has been shown to (i) significantly attenuate pathological elevation of critical inflammatory cell signaling pathways (NF-κB), (ii) decrease the resulting elevated proinflammatory cytokine levels, (iii) reduce immune cell infiltration of the lung, and (iv) positively influence macrophage polarization in humans and animal models of disease.
| 10 |
Our Products and Business Strategy
Our product platform consists of our development stage pharmaceutical candidates and our commercially available dietary supplement:
| ● | CDX-101, our lead pharmaceutical candidate, is in pre-clinical development for cardiovascular inflammation and dyslipidemia, with a target initial indication of severe hypertriglyceridemia. | |
| ● | CDX-301 is in pre-clinical development for macular degeneration, with a target initial indication of Stargardt disease. | |
| ● | ZanthoSyn® is a physician recommended astaxanthin dietary supplement for inflammatory health. |
Lead Pharmaceutical Candidate: CDX-101
Our lead pharmaceutical candidate, CDX-101, is a proprietary astaxanthin prodrug that cleaves following oral administration and delivers astaxanthin to the bloodstream. CDX-101 is being developed initially for cardiovascular inflammation and mixed dyslipidemia, with a target initial indication of severe hypertriglyceridemia.
We believe that the results from two major cardiovascular clinical trials—the 10,061 patient CANTOS study by Novartis in 2017 and the 8,179 patient REDUCE-IT study by Amarin in 2018—clearly demonstrated the clinical significance of reducing chronic inflammation, validating the cardiovascular inflammation hypothesis we have supported for more than a decade. We believe that astaxanthin’s unique mechanism of action—reduction of oxidative stress driven inflammation at the cellular and mitochondrial level without inhibiting normal function—results in an impact on key inflammatory drug targets and pathways, and importantly, an excellent safety profile that supports chronic administration. In addition to the safety advantages described in this report, we believe that production of CDX-101, unlike Vascepa and other prescription fish oil drugs, will be highly scalable to address these large mass markets for chronic diseases driven by inflammation.
Clinical and non-clinical studies with astaxanthin have provided proof-of-concept for the treatment of cardiovascular risk factors including inflammation and triglycerides as described in this report. In addition, interim results from our Cardiovascular Health Astaxanthin Supplement Evaluation (“CHASE”) clinical trial demonstrate beneficial changes in markers of cardiovascular health, including CRP, LDL cholesterol, total cholesterol, triglycerides, oxidized LDL, and blood pressure, and also underscore astaxanthin’s safety profile with no adverse safety signals observed. We believe these findings provide further mechanistic support for our pharmaceutical development program. We refer you to “CHASE Clinical Trial” in this report for additional information regarding the CHASE clinical trial.
We believe that an initial indication of severe hypertriglyceridemia provides an efficient clinical pathway to drug approval for CDX-101 and will be similar to the pathway as reported by Amarin for the development of Vascepa, its prescription fish oil. CDX-101 is currently in pre-clinical development, including the planning of IND enabling studies. We plan to use proceeds from the Proposed Public Offering to complete IND enabling studies and to engage third party contract development and manufacturing organizations (CDMOs) to manufacture drug substance and drug product for such studies, with the goal of filing an IND approximately one year from the closing of the Proposed Public Offering.
| 11 |
We have retained Paresh N. Soni, M.D., Ph.D., the former Senior Vice President and Head of Development at Amarin, to guide our clinical and regulatory strategy, interact with the FDA, and advise us on a full range of development issues. While at Amarin, Dr. Soni led the design of Amarin’s clinical trials, development strategy, and interaction with the FDA, including for Vascepa, which was approved for treatment of severe hypertriglyceridemia in 2012. Dr. Soni played a key role in the design and conduct of the MARINE, ANCHOR and REDUCE-IT clinical trials with Vascepa. In addition, Dr. Soni has held several senior R&D executive roles over the past 2 decades at Pfizer, Alexion, and Albireo. Dr. Soni is also a member of our Scientific Advisory Board.
In addition to Dr. Soni, our Scientific Advisory Board includes Deepak L. Bhatt, M.D., M.P.H. and R. Preston Mason, Ph.D.
Deepak L. Bhatt, M.D., M.P.H., is the Chairman of our Scientific Advisory Board. Dr. Bhatt is also the Chair of the REDUCE-IT clinical trial with Vascepa, Executive Director of Interventional Cardiovascular Programs at Harvard Medical School affiliated Brigham and Women’s Hospital, and Professor at Harvard Medical School. He is also the Editor of the peer-reviewed Journal of Invasive Cardiology and Editor-in-Chief of the Harvard Heart Letter for patients.
R. Preston Mason, Ph.D. is on the faculty of the Department of Medicine, Division of Cardiology at Harvard Medical School affiliated Brigham and Women’s Hospital. He has published more than 250 peer reviewed papers, including papers published in collaboration with Cardax, and is a recognized expert on the mechanism of action of astaxanthin and fish oils, particularly Vascepa.
CDX-101 vs. ZanthoSyn®
CDX-101 is a synthetic astaxanthin prodrug (new chemical entity) for pharmaceutical applications and ZanthoSyn® is a formulation of synthetic nature-identical astaxanthin for dietary supplement applications. While both deliver astaxanthin to the bloodstream, we believe the unique molecular structure of CDX-101 and its pharmaceutical pathway will provide substantial differentiation. In particular, we believe that:
| ● | CDX-101 will be approved by the FDA as a drug for one or more disease indications, whereas ZanthoSyn® is marketed as a dietary supplement for health applications; | |
| ● | CDX-101 will be prescribed by doctors and covered by health insurance, whereas ZanthoSyn® is sold through retail and e-commerce channels; | |
| ● | CDX-101 will be administered at a higher dose and in different oral dosage form; and | |
| ● | CDX-101 will have superior intellectual property protection. |
Pharmaceutical Candidate: CDX-301
Our zeaxanthin pharmaceutical candidate, CDX-301, has a mechanism of action and excellent safety profile similar to astaxanthin, however, it is being developed for macular degeneration because zeaxanthin accumulates in the human eye through uptake by a unique retinal receptor, providing protection against blue light, oxidative damage, and related inflammation that occurs in macular degeneration. Pre-clinical and clinical studies with zeaxanthin have demonstrated proof-of-concept for the treatment of macular disorders. We believe that an initial indication of Stargardt disease, a juvenile form of macular degeneration, provides an efficient clinical pathway to drug approval for CDX-301. On November 30, 2018, we submitted a request for orphan drug designation to the FDA for zeaxanthin as a treatment of Stargardt disease, and we are currently in communications with the FDA regarding this matter. Additional financing beyond that contemplated in the Proposed Public Offering will be needed to fund IND enabling studies and clinical development of CDX-301.
| 12 |
Dietary Supplement: ZanthoSyn®
ZanthoSyn® is our commercially available physician recommended astaxanthin dietary supplement. Astaxanthin is a naturally occurring molecule with safe anti-inflammatory activity that supports cardiovascular health, metabolic health, liver health, joint health, and longevity. The form of astaxanthin utilized in ZanthoSyn® has demonstrated an excellent safety profile in peer-reviewed published studies and is GRAS according to FDA regulations.
We sell ZanthoSyn® primarily through wholesale and e-commerce channels. We launched our e-commerce channel in 2016 and began selling to GNC stores in 2017. ZanthoSyn® is currently available at GNC corporate stores nationwide in the United States.
We market ZanthoSyn® primarily through a multi-pronged approach:
| ● | Physician outreach and education, where ZanthoSyn® is positioned as the first safe, physician friendly, anti-inflammatory dietary supplement for health and longevity, with retail locations and e-commerce serving as convenient and credible distribution channels for physicians recommending ZanthoSyn® | |
| ● | Retail store outreach, education, and in-store sales support, building on the ability to utilize ZanthoSyn® as a foundation of health and wellness regimens | |
| ● | E-commerce platforms |
We believe ZanthoSyn® is physician friendly for several reasons:
| ● | ZanthoSyn® delivers the safety, purity, manufacturing rigor, bioavailability, and scientific support that provides physicians comfort in the quality and utility of the product, which is often not present in other dietary supplements. | |
| ● | ZanthoSyn® is well-accepted at medical conferences where crowds of physicians and other healthcare professionals stand in line to receive ZanthoSyn® samples and product information after attending educational seminars. |
Our sales and marketing program was initially launched in Hawaii, where we believe that robust physician outreach and education coupled with GNC retail store outreach, education, and in-store sales support increased consumer awareness and catalyzed strong sales growth. We also launched this program in major markets on the West Coast and East Coast in the U.S. beginning in 2017. To support these efforts, we have hired additional sales and marketing personnel. We are currently evaluating our strategy related to further expansion.
We sell ZanthoSyn® to GNC under a purchasing agreement. The exclusivity provision under such agreement related to distribution of ZanthoSyn® by GNC in the “brick and mortar” retail channel in the United States expired on October 16, 2019. GNC remains our only distributor of ZanthoSyn® in such channel, but we may expand retail distribution to mass market retailers, other specialty nutrition stores, pharmacies, and other retailers. We also plan to increase our sales and marketing efforts through e-commerce.
To date, our sales and marketing efforts of ZanthoSyn® have primarily been through GNC retail store outreach, education, and in-store sales support together with physician outreach and education. We plan to increase our sales and marketing efforts through e-commerce by capitalizing on one of the most important lessons learned from our sales and marketing program: “Conversations Create Customers.” Whether at GNC stores, directly with Cardax personnel, or at conferences with healthcare professionals, thousands of ZanthoSyn® customers have been created by understanding and experiencing the benefits of ZanthoSyn® firsthand. Cardax plans to implement strategies that it believes may create a similar customer experience more broadly, with fulfillment online, where margins may be greater than retail stores.
| 13 |
CHASE Clinical Trial
In September 2018, we initiated a human clinical trial entitled, Cardiovascular Health Astaxanthin Supplement Evaluation (“CHASE”), targeting cardiovascular inflammatory health. The randomized, double-blind, placebo-controlled clinical trial is evaluating the effect of low-dose and high-dose ZanthoSyn® on cardiovascular health as measured by CRP levels over 12 weeks in up to 120 subjects with documented cardiovascular risk factors. The study also includes an optional open label extension through 48 weeks.
Interim results from an initial cohort of subjects were announced on September 23, 2019. The interim results were based on data from 40 subjects administered high-dose ZanthoSyn® (96 mg/day astaxanthin – 48 mg twice a day), low-dose ZanthoSyn® (24 mg/day astaxanthin – 12 mg twice a day), or placebo.
Highlights from the interim review shown below are median percentage changes from baseline to week 12 unless otherwise stated. While the interim review was not powered for statistical significance, p-values less than 0.05 compared to placebo are provided. The p-values reported below (*p<0.05, **p<0.01) are nominal p-values from non-parametric comparisons of the median between each group and placebo and no adjustments for multiple comparisons were made.
| Interim Results | High Dose | Low Dose | Placebo | |||||||||
| CRP | -28 | % | -32 | % | -5 | % | ||||||
| LDL-C | -12 | %** | -7 | % | +5 | % | ||||||
| Total cholesterol | -8 | %* | -5 | % | +4 | % | ||||||
| Triglycerides | -16 | % | -13 | % | +6 | % | ||||||
| Oxidized LDL | -10 | %* | +3 | % | +4 | % | ||||||
| Blood pressure | -5 | %* | -4 | %* | +6 | % | ||||||
| Median astaxanthin blood levels at 12 weeks | 2,184 ng/mL | 790 ng/mL | <10 ng/mL | |||||||||
We believe these findings provide:
| ● | Further mechanistic support for our astaxanthin pharmaceutical development program | |
| ● | Basis for additional patent filings | |
| ● | Support for the cardiovascular health benefits of ZanthoSyn® |
The interim results also underscore astaxanthin’s safety profile with no adverse safety signals observed. The CHASE Data Safety Review Board, which is comprised of a majority of independent clinical trial professionals, recommended that the clinical trial continue enrollment.
The FDA does not require human clinical trials for dietary supplements, but we believe that positive results from the CHASE trial may help promote scientific and consumer awareness of astaxanthin’s health and longevity applications and serve as further mechanistic support for our pharmaceutical development program.
We refer to you the “Risk Factors” section of this report for a summary of certain risks related to clinical trial results.
| 14 |
Benefits of Synthetic Astaxanthin vs. Natural Astaxanthin
Dietary supplements containing astaxanthin typically derive astaxanthin from microalgae, krill, or other natural sources, whereas ZanthoSyn® astaxanthin is made through total synthesis. While multiple studies demonstrate that astaxanthin from either natural or synthetic sources is efficacious and both are Generally Recognized as Safe according to FDA regulations, we believe synthetic astaxanthin offers significant advantages compared to astaxanthin from microalgae, krill, or other natural sources:
| ● | Synthetic astaxanthin can be formulated for superior bioavailability. In a human crossover study comparing ZanthoSyn® to a leading microalgal astaxanthin dietary supplement, the astaxanthin blood levels following administration of ZanthoSyn® were nearly three times higher than the microalgal astaxanthin product at the same dose: |
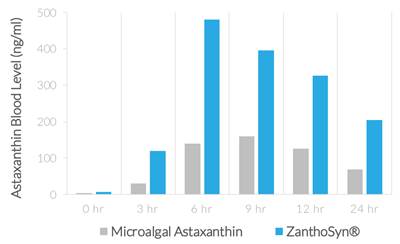
| ● | AUC (area under curve, astaxanthin blood levels) = 2.85-fold greater (p=0.013) | |
| ● | Cmax (maximum concentration, astaxanthin blood levels) = 3.0-fold greater (p=0.013) | |
| ● | Coefficient of variation (variation between subjects of astaxanthin blood levels) |
| ○ | ZanthoSyn® = 27% | |
| ○ | Microalgal astaxanthin = 62% |
| ● | Tmax (time of maximum concentration) = 6 hours | |
| ● | No adverse events observed |
The superior bioavailability described in this report means that three times more astaxanthin from ZanthoSyn® is absorbed into the body from each dose, which provides a superior value proposition compared to other astaxanthin dietary supplements.
| ● | Synthetic astaxanthin has been extensively tested in a wide range of toxicity studies, including acute, subacute, sub-chronic, and chronic toxicity studies, carcinogenicity studies, genotoxicity/mutagenicity studies, and developmental and reproductive toxicity studies; whereas to our knowledge microalgal or other sources of astaxanthin have not undergone the same amount of safety testing in such toxicity studies. | |
| ● | Synthetic astaxanthin is manufactured with superior purity and precision, whereas astaxanthin extracted from microalgae and krill oil is obtained in a complex mixture, which may include many unknown marine byproducts. | |
| ● | Synthetic manufacture of astaxanthin is scalable, whereas we believe the ability to readily scale the production and extraction of astaxanthin from microalgae or other sources will be limited as demand for astaxanthin grows. | |
| ● | Synthetic manufacture of astaxanthin emits fewer greenhouse gases and consumes less energy, raw material, and land than traditional microalgal astaxanthin production. |
| 15 |
Intellectual Property
We have obtained and are continuing to seek patent protection for compositions of matter, pharmaceutical compositions, and pharmaceutical uses, in certain disease areas, of our various carotenoid analogs and derivatives. Such carotenoids include astaxanthin, zeaxanthin, lutein, and/or lycophyll, and esters and other analogs and derivatives of these compounds. More specifically, we seek to protect: (i) the composition of matter of novel carotenoid analogs and derivatives, (ii) pharmaceutical compositions comprising synthetic or natural preparations of novel or natural occurring carotenoid analogs and derivatives, and (iii) the pharmaceutical use of synthetic preparations of novel or naturally occurring carotenoid analogs and derivatives in specific disease areas, including, but not limited to, the treatment of inflammation and related tissue damage, liver disease, and reperfusion injury, as well as the pharmaceutical use of synthetic or natural preparations of novel or natural occurring carotenoid analogs and derivatives for the reduction of platelet aggregation. We intend to enforce and defend our intellectual property rights consistent with our strategic business objectives.
We have 29 issued patents and one pending patent related to the composition of matter, pharmaceutical compositions, and pharmaceutical uses of our drugs candidates as well as many other related molecules that will expire between 2023 and 2028, subject to patent term extensions. We also have filed four additional patents that if issued would extend patent coverage in the U.S. and worldwide to 2039-2041, with such applications related to (i) certain cardiovascular uses on the basis of the CHASE clinical trial results, (ii) certain uses related to the potential role of astaxanthin in the treatment of COVID-19, and (iii) the composition of matter of CDX-101.
The Company’s patents are summarized in the table below.
| United States | Foreign | Expiration | ||||||||||
| Issued Patents | 14 | 15 | 2023-2028 | |||||||||
| Pending Patents | 0 | 1 | 2023-2028 | |||||||||
| Pending Patents | 3 | 1 | 2039-2041 | |||||||||
Research and Development
Our research and development program is presently comprised of employees, consultants, including regulatory, scientific, and medical professionals, and third-party collaborators or contract organizations, including academic institutions, contract research organizations, and contract development and manufacturing organizations. Contract organizations provide us with access to significant research and development resources and infrastructure. We anticipate that our research and development will be primarily conducted by contract organizations with direction and oversight by our in-house research and development personnel.
In addition to conducting or overseeing research and development activities, our research and development personnel analyze and interpret other research on astaxanthin, as well as related compounds, competing products, applications, and industry trends. In the United States National Library of Medicine’s online repository, PubMed.gov, there are more than 2,000 peer-reviewed journal articles that reference astaxanthin in the title or abstract, over 500 of which were published in the last three years, with the vast majority published by organizations and researchers that are not affiliated with us. This type of “open-source” research has served to significantly advance the understanding of astaxanthin and related carotenoids, and has also presented our research and development personnel with the critical task of keeping up-to-date on all of the latest research and interpreting and integrating the findings with our research and that of others in order to serve as leading experts on the mechanism of action and biological applications of astaxanthin and related carotenoids.
Our research and development expenditures totaled $315,994 and $269,077 for the years ended December 31, 2019 and 2018, respectively. These expenditures primarily reflect the cost of product development activities, including clinical trials. The compensation of our research and development personnel are included as a component of salaries and wages in the consolidated statements of operations.
| 16 |
Manufacturing
We utilize contract manufacturers and/or other third-party suppliers for the production of our products. The raw materials and supplies required for the production of our products may be available, in some instances from one supplier, and in other instances, from multiple suppliers. In those cases where raw materials are only available through one supplier, such supplier may be either a sole source (the only recognized supply source available to us) or a single source (the only approved supply source for us among other sources). We, our contract manufacturers, and/or other third-party suppliers will adopt appropriate policies to attempt, to the extent feasible, to minimize our raw material supply risks, including maintenance of greater levels of raw materials inventory and implementation of multiple raw materials sourcing strategies, especially for critical raw materials. Although to date we have not experienced any significant delays in obtaining any raw materials from suppliers, we cannot provide assurance that we, our contract manufacturers, and/or other third-party suppliers will not face shortages from one or more suppliers in the future.
Competition
The industries in which we intend to compete are subject to intense competition. The primary competition for our pharmaceutical candidates are the numerous pharmaceutical and biotechnology companies developing or marketing anti-inflammatories and other drugs or therapeutics targeting chronic diseases driven by inflammation, including cardiovascular disease, metabolic disease, liver disease, arthritis, aging, and macular degeneration. Certain competitors for our pharmaceutical candidates include, but are not limited to, AbbVie, Acasti Pharma, Acucela, Alkeus Pharmaceuticals, Amarin, Amgen, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, Johnson & Johnson, Matinas Biopharma, Merck, MT Pharma, Nestle/Pamlab, Novartis, Pfizer, Reata Pharmaceuticals, Regeneron Pharmaceuticals, Roche/Genentech, Sanofi-Aventis, Servier, and Takeda. The primary competition for our dietary supplements are the many companies developing or marketing astaxanthin dietary supplements and other supplements targeting inflammatory health, cardiovascular health, metabolic health, liver health, joint health, and longevity. We believe that our ability to compete will be based primarily on scientific superiority, availability of patent protection, protection of other intellectual property rights, access to adequate capital, ability to develop, acquire, and market products successfully, ability to obtain governmental approvals, and ability to serve the particular needs of customers.
Our success will depend in large part on our ability to obtain and maintain international and domestic patents, other intellectual property, and other legal protections for the proprietary technology that we consider important to our business. We intend to continue to seek appropriate patent protection for our products where applicable by filing patent applications in the United States and other selected countries. We intend for these patent applications to cover, where applicable, claims for composition of matter, uses, manufacturing processes, and formulations. Our success will also depend on our ability, and the ability of our current and/or future strategic partners to maintain intellectual property rights related to proprietary production methods for products that we, or our partners, intend to market.
| 17 |
Government Regulation
Most aspects of our business are subject to some degree of government regulation. For some of our products, government regulation is significant and, in general, there appears to be a trend toward more stringent regulation throughout the world, as well as global harmonization of various regulatory requirements. We expect to devote significant time, effort and expense to address the extensive government and regulatory requirements applicable to our business. We believe that we are no more or less adversely affected by existing government regulations than our competitors.
FDA Regulation
Biopharmaceutical companies must comply with comprehensive regulation by the FDA and other regulatory agencies in the United States and comparable authorities in other countries. While the FDA does not require human clinical trials for dietary supplements, we have conducted and may continue to conduct clinical trials with our dietary supplements to promote scientific and consumer awareness. We may also conduct Phase I, Phase II, and/or Phase III clinical trials with our pharmaceutical candidates.
We must obtain regulatory approvals by the FDA and similar health authorities in foreign countries to the extent applicable prior to human clinical testing and marketing of any pharmaceutical and for post-approval clinical studies for additional indications of approved drugs. We anticipate that any pharmaceutical candidate will be subject to rigorous preclinical and clinical testing and pre-market approval procedures by the FDA and similar health authorities in foreign countries to the extent applicable. The extent to which our products are regulated by the FDA will depend upon the types of products we ultimately develop. We are currently evaluating and pursuing various developmental strategies and cannot predict, during this stage of our development, the scope of FDA or other agency regulation to which we or our products will be subject. Various federal statutes and regulations also govern or influence the preclinical and clinical testing, record-keeping, approval, labeling, manufacture, quality, shipping, distribution, storage, marketing and promotion, export, and reimbursement of pharmaceuticals.
The steps ordinarily required before a drug product may be marketed in the United States include:
| ● | preclinical studies; | |
| ● | submission to the FDA of an investigational new drug (“IND”) application, which must become effective before human clinical trials may commence; | |
| ● | adequate and well-controlled human clinical trials to establish the safety and efficacy of the pharmaceutical candidate in the desired indication for use; | |
| ● | submission to the FDA of a new drug application (“NDA”), together with payment of a substantial user fee; and | |
| ● | FDA approval of the NDA, including inspection and approval of the product manufacturing facility and select sites at which human clinical trials were conducted. |
Preclinical studies typically involve laboratory evaluation of pharmaceutical candidate chemistry, formulation, and stability, as well as animal studies to assess the potential safety and efficacy of the pharmaceutical candidate. The results of preclinical studies are submitted to the FDA as part of an IND and are reviewed by the FDA before the commencement of clinical trials. Unless the FDA objects to an IND, the IND will become effective 30 days following its receipt by the FDA. Submission of an IND may not result in FDA clearance to commence clinical trials, and the FDA’s failure to object to an IND does not guarantee FDA approval of a marketing application.
| 18 |
Clinical trials involve the administration of the test agent to humans under the supervision of a qualified principal investigator. In the United States, clinical trials must be conducted in accordance with Good Clinical Practices. In addition, each clinical trial must be approved and conducted under the auspices of an institutional review board and with the subject’s informed consent. We would be subject to similar regulatory considerations if we conduct clinical trials outside the United States.
The goal of Phase I clinical trials is to establish initial data about safety and tolerability of the pharmaceutical candidate in humans. The investigators seek to evaluate the effects of various dosages and to establish an optimal dosage level and schedule.
The goal of Phase II clinical trials is to provide evidence about the desired therapeutic efficacy of the pharmaceutical candidate in limited studies with small numbers of carefully selected subjects. Investigators also gather additional safety data.
Phase III clinical trials consist of expanded, large-scale, multi-center studies in the target patient population. This phase further tests the product’s effectiveness, monitors side effects, and, in some cases, compares the product’s effects to a standard treatment, if one is already available. Phase III trials are designed to more rigorously test the efficacy of a pharmaceutical candidate and are normally randomized, double-blinded, and placebo-controlled. Phase III trials are typically monitored by an independent data monitoring committee, or DMC, which periodically reviews data as a trial progresses. A DMC may recommend that a trial be stopped before completion for a number of reasons including safety concerns, patient benefit, or futility.
Data obtained from this development program are submitted as part of an NDA to the FDA and possibly to corresponding agencies in other countries for review. The NDA requires agency approval prior to marketing in the relevant country. Extensive regulations define the form, content and methods of gathering, compiling and analyzing the pharmaceutical candidate’s safety and efficacy data.
The process of obtaining regulatory approval can be costly, time consuming and subject to unanticipated delays. Regulatory agencies may refuse to approve an application if they believe that applicable regulatory criteria are not satisfied and may also require additional testing for safety and efficacy and/or post-marketing surveillance or other ongoing requirements for post-marketing studies. In some instances, regulatory approval may be granted with the condition that confirmatory Phase IV clinical trials are carried out, and if these trials do not confirm the results of previous studies, regulatory approval for marketing may be withdrawn. Moreover, each regulatory approval of a product is limited to specific indications. The FDA or other regulatory authorities may approve only limited label information for the product. The label information describes the indications and methods of use for which the product is authorized, may include Risk Evaluation and Mitigation Strategies and, if overly restrictive, may limit a sponsor’s ability to successfully market the product. Regulatory agencies routinely revise or issue new regulations, which can affect and delay regulatory approval of pharmaceuticals.
Furthermore, pharmaceutical manufacturing processes must conform to current Good Manufacturing Practices, or cGMPs. Manufacturers, including a drug sponsor’s third-party contract manufacturers, must expend time, money and effort in the areas of production, quality control and quality assurance, including compliance with stringent record-keeping requirements. Manufacturing establishments are subject to periodic inspections by the FDA or other health authorities, in order to assess, among other things, compliance with cGMP. Before approval of the initiation of commercial manufacturing processes, the FDA will usually perform a preapproval inspection of the facility to determine its compliance with cGMP and other rules and regulations. In addition, foreign manufacturers must also comply with cGMPs in order to supply products for use in the United States, and are subject to periodic inspection by the FDA or by regulatory authorities in certain countries under reciprocal agreements with the FDA. Manufacturing processes and facilities for pharmaceuticals are highly regulated. Regulatory authorities may choose not to certify or may impose restrictions, or even shut down existing manufacturing facilities that they determine are non-compliant.
| 19 |
FDA GRAS Determination
“GRAS” is an acronym for the phrase “generally recognized as safe,” which the FDA utilizes to describe those substances that, in the generally recognized opinion of the scientific community, will not be harmful to consumers, provided the substance is used as intended. According to applicable FDA regulations, any substance that is intentionally added to food is a food additive, which is subject to premarket review and approval by FDA, unless the substance is generally recognized, among qualified experts, as having been adequately shown to be safe under the conditions of its intended use. Under sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act (the “FD&C Act”), and FDA’s implementing regulations in 21 CFR 170.3 and 21 CFR 170.30, the use of a food substance may be GRAS either through scientific procedures or, for a substance used in food before 1958, through experience based on common use in food. General recognition of safety through scientific procedures requires the same quantity and quality of scientific evidence as is required to obtain approval of the substance as a food additive and ordinarily is based upon published studies, which may be corroborated by unpublished studies and other data and information. General recognition of safety through experience based on common use in foods requires a substantial history of consumption for food use by a significant number of consumers.
Manufacturers of GRAS substances may provide the FDA with a notification of GRAS determination, which includes a description of the substance, the applicable conditions of use, and an explanation of how the substance was determined to be safe. Upon review of such a notification, the FDA may respond with a “no questions” position, whereby the manufacturer’s determination that a product is GRAS for its intended purposes is affirmed. Alternatively, manufacturers may elect to “self-affirm” a given substance as GRAS without FDA notification but should retain all applicable safety data used for GRAS determination in the case of FDA inquiry.
Synthetic copies of naturally-occurring dietary ingredients or related components do not qualify as dietary ingredients under the FD&C Act, but substances that have been affirmed by the FDA as GRAS, self-affirmed as GRAS, or approved as direct food additives in the U.S. may be marketed as dietary ingredients, subject to FDA regulations for dietary ingredients.
FDA NDI Notification
The Dietary Supplement Health and Education Act of 1994 (the “DSHEA”) (Pub. L. 103-417) was signed into law on October 25, 1994 and amended the FD&C Act by adding: (i) section 201(ff) (21 U.S.C. 321(ff)), which defines the term “dietary supplement”, and (ii) section 413 (21 U.S.C. 350b), which defines the term “new dietary ingredient” (“NDI”) and requires the manufacturer or distributor of an NDI, or of the dietary supplement that contains the NDI, to submit a premarket notification to FDA at least 75 days before introducing/delivering the supplement into interstate commerce, unless the NDI and any other dietary ingredients in the dietary supplement have been present in the food supply without chemical alteration (21 U.S.C. 350b(a)(1)). The NDI notification must contain applicable information, including history of use and citations to published articles, from which the manufacturer or distributor of the NDI or dietary supplement has concluded that the dietary supplement containing the NDI will be reasonably expected to be safe under the conditions of its intended use. NDI notifications are not required for the marketing of approved food additives or GRAS substances as NDIs unless the dietary ingredient has been chemically altered.
| 20 |
FDA Orphan Drug Designation
The Orphan Drug Act was signed into law on January 4, 1983. The Congressional findings for the Orphan Drug Act were as follows: (i) there are many rare diseases and conditions that affect such small numbers of individuals residing in the United States; (ii) adequate drugs for many rare diseases and conditions have not been developed; (iii) drugs for rare diseases and conditions are commonly referred to as “orphan drugs”; (iv) because so few individuals are affected by any one rare disease or condition, a pharmaceutical company that develops an orphan drug may reasonably expect the drug to generate relatively small sales in comparison to the cost of developing the drug and consequently to incur a financial loss; (v) there is reason to believe that some promising orphan drugs will not be developed unless changes are made in the applicable Federal laws to reduce the costs of developing such drugs and to provide financial incentives to develop such drugs; and (vi) it is in the public interest to provide such changes and incentives for the development of orphan drugs.
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition that (i) affects less than 200,000 persons in the United States, or (ii) affects more than 200,000 in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for such disease or condition will be recovered from sales in the United States of such drug. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the identity of the drug and its potential orphan use are disclosed publicly by the FDA.
In the United States, orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages, and NDA user-fee waivers. In addition, if a drug receives the first FDA approval for the indication for which it has orphan designation, the drug is entitled to orphan drug exclusivity, which means the FDA may not approve any other application, including a full NDA, to market the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority over the drug with orphan exclusivity or where the manufacturer with orphan exclusivity is unable to assure sufficient quantities of the approved orphan-designated drug. Competitors, however, may receive approval of different drugs for the indication that the orphan drug has exclusivity or obtain approval for the same drug but for a different indication for which the orphan drug has exclusivity. Orphan drug exclusivity also could block the approval of one of our drugs for seven years if a competitor obtains approval of the same drug as defined by the FDA or if our drug is determined to be contained within the competitor’s drug for the same indication or disease. If a drug designated as an orphan drug receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan drug exclusivity. In addition, exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the drug to meet the needs of patients with the rare disease or condition. There can be no assurance that any request for orphan drug designation will be granted by the FDA.
| 21 |
Other Regulations
Pharmaceutical companies are subject to various federal and state laws pertaining to healthcare “fraud and abuse,” including anti-kickback and false claims laws. The Anti-Kickback Statute is a federal criminal statute that makes it illegal for any person, including a prescription drug manufacturer, or a party acting on its behalf, to knowingly and willfully solicit, offer, receive or pay any remuneration, directly or indirectly, in exchange for, or to induce, the referral of business, including the purchase, order or prescription of a particular drug, for which payment may be made under federal healthcare programs such as Medicare and Medicaid. Some of the state prohibitions apply to referral of patients for healthcare services reimbursed by any source, not only the Medicare and Medicaid programs.
In the course of practicing medicine, physicians may legally prescribe FDA approved drugs for an indication that has not been approved by the FDA and which, therefore, is not described in the product’s approved labeling, so-called “off-label use.” The FDA does not ordinarily regulate the behavior of physicians in their choice of treatments. The FDA and other governmental agencies do, however, restrict communications on the subject of off-label use by a manufacturer or those acting on behalf of a manufacturer. Companies may not promote FDA-approved drugs for off-label uses. The FDA and other governmental agencies do permit a manufacturer (and those acting on its behalf) to engage in some limited, non-misleading, non-promotional exchanges of scientific information regarding unapproved indications. The United States False Claims Act prohibits, among other things, anyone from knowingly and willfully presenting, or causing to be presented for payment to third-party payers (including Medicare and Medicaid) claims for reimbursed drugs or services that are false or fraudulent, claims for items or services not provided as claimed or claims for medically unnecessary items or services. Violations of fraud and abuse laws may be punishable by criminal and/or civil sanctions, including imprisonment, fines and civil monetary penalties, as well as possible exclusion from federal health care programs (including Medicare and Medicaid). In addition, under this and other applicable laws, such as the Food, Drug and Cosmetic Act, there is an ability for private individuals to bring similar actions. Further, there is an increasing number of state laws that require manufacturers to make reports to states on pricing and marketing information. Many of these laws contain ambiguities as to what is required to comply with the law.
We are subject to various laws and regulations regarding laboratory practices and the experimental use of animals in connection with our research. In each of these areas, as above, the FDA and other regulatory authorities have broad regulatory and enforcement powers, including the ability to suspend or delay issuance of approvals, seize or recall products, withdraw approvals, enjoin violations and institute criminal prosecution, any one or more of which could have a material adverse effect upon our business, financial condition, and results of operations.
We must comply with regulations under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act and other federal, state and local regulations. We are subject to federal, state and local laws and regulations governing the use, generation, manufacture, storage, air emission, effluent discharge, handling and disposal of certain hazardous or potentially hazardous materials. We may be required to incur significant costs to comply with environmental and health and safety regulations in the future. Our research and development involves the controlled use of hazardous materials, including, but not limited to, certain hazardous chemicals.
Our activities are also potentially subject to federal and state consumer protection and unfair competition laws. We are also subject to the United States Foreign Corrupt Practices Act, or the FCPA, which prohibits companies and individuals from engaging in specified activities to obtain or retain business or to influence a person working in an official capacity. Under the FCPA, it is illegal to pay, offer to pay, or authorize the payment of anything of value to any foreign government official, governmental staff members, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity. In addition, federal and state laws protect the confidentiality of certain health information, in particular, individually identifiable information, and restrict the use and disclosure of that information. At the federal level, the Department of Health and Human Services promulgated health information privacy and security rules under the Health Insurance Portability and Accountability Act of 1996. In addition, many state laws apply to the use and disclosure of health information.
| 22 |
Customers
We sell ZanthoSyn® primarily through wholesale and e-commerce channels. We launched our e-commerce channel in 2016, and we began selling to GNC stores in 2017. ZanthoSyn® is currently available at over two thousand GNC corporate stores in the United States.
During the years ended December 31, 2019 and 2018, sales to GNC accounted for approximately 90% of our revenues. No other customer accounted for 10% or more of our revenues during these years.
We sell ZanthoSyn® to GNC under a purchasing agreement. The exclusivity provision under such agreement related to distribution of ZanthoSyn® by GNC in the “brick and mortar” retail channel in the United States expired on October 16, 2019. GNC remains our only distributor of ZanthoSyn® in such channel, but we may expand retail distribution to mass market retailers, other specialty nutrition stores, pharmacies, and other retailers. We also plan to increase our sales and marketing efforts through e-commerce.
Employees
As of the date of this report, we have 11 full-time employees and 1 part-time employee. None of our employees are subject to a collective bargaining agreement. We believe the relations with our employees are satisfactory.
| 23 |
An investment in our common stock, any warrants to purchase our common stock, or any other security that may be issued by us involves a high degree of risk. You should carefully consider the risks described below, together with all of the other information included elsewhere in this annual report, before making an investment decision. If any of the following risks actually occur, our business, financial condition, or results of operations could suffer. In that case, the trading price of our shares of common stock could decline, and you may lose all or part of your investment. You should read the section entitled “Forward-Looking Statements” above for a discussion of what types of statements are forward-looking statements, as well as the significance of such statements in the context of this annual report.
Risks Related to Our Business, Industry, and Financial Condition
We have a history of operating losses and have received a going concern opinion from our auditors.
We have incurred substantial net losses since our inception and may continue to incur losses for the foreseeable future, as we continue our product development activities. As a result of our limited operating history, we have limited historical financial data that can be used in evaluating our business and our prospects and in projecting our future operating results. Through December 31, 2019, we have accumulated a total deficit of $67,036,355.
Additionally, we have received a “going concern” opinion from our independent registered public accounting firm. We expect that our marketing program for ZanthoSyn® will continue to focus on outreach to physicians, healthcare professionals, retail personnel, and consumers, and anticipate further losses in the development of our consumer business. We also plan to advance the research and development of our pharmaceutical candidates and anticipate further losses in the development of our pharmaceutical business. As a result of these and other factors, management has determined there is substantial doubt about the Company’s ability to continue as a going concern. Our ability to continue as a going concern is dependent upon our ability to raise additional capital and implement our business plan. If we are unable to achieve or sustain profitability or to secure additional financing on acceptable terms, we may not be able to meet our obligations as they come due, raising substantial doubts as to our ability to continue as a going concern. Any such inability to continue as a going concern may result in our common stock holders losing their entire investment. There is no guarantee that we will become profitable or secure additional financing on acceptable terms. Our consolidated financial statements contemplate that we will continue as a going concern and do not contain any adjustments that might result if we were unable to continue as a going concern. Changes in our operating plans, our existing and anticipated working capital needs, the acceleration or modification of our expansion plans, increased expenses, potential acquisitions or other events will all affect our ability to continue as a going concern.
| 24 |
We are highly dependent on our senior management and certain consultants or other advisors, and if we are not able to retain them or to recruit and retain additional qualified personnel, our business will suffer.
We are highly dependent upon our senior management and certain consultants or other advisors, including David G. Watumull, our President and Chief Executive Officer, David M. Watumull, our Chief Operating Officer, Paresh N. Soni, our Chief Clinical and Regulatory Strategist, Gilbert M. Rishton, our Chief Science Officer, Jon L. Ruckle, our Chief Medical Officer, Timothy J. King, our Vice President, Research, and John B. Russell, our Chief Financial Officer. The loss of services of David G. Watumull or any other member of our senior management could have a material adverse effect on our business, prospects, financial condition, and results of operations. We carry $1 million “key person” life insurance policies on David G. Watumull and David M. Watumull, but we do not carry similar insurance for any of our other senior executives.
We may choose to increase our management personnel. For example, we will need to obtain certain additional functional capability, including regulatory, sales, quality assurance and control, either by hiring additional personnel or by outsourcing these functions to qualified third parties. We may not be able to engage these third parties on terms favorable to us. Also, we may not be able to attract and retain qualified personnel on acceptable terms given the competition for such personnel among companies that operate in our markets. The trend in the pharmaceutical industry of requiring sales and other personnel to enter into non-competition agreements prior to starting employment exacerbates this problem, since personnel who have made such a commitment to their current employers are more difficult to recruit. If we fail to identify, attract, retain, and motivate these highly skilled personnel, or if we lose current employees, our business, prospects, financial conditions, and results of operations could be adversely affected.
Our ability to grow and compete in the future will be adversely affected if adequate capital is not available to us or not available on terms favorable to us.
The ability of our business to grow and compete depends on the availability of adequate capital, which in turn depends in large part on our cash flow from operations and the availability of equity and debt financing. We cannot assure you that our cash flow from operations will be sufficient or that we will be able to obtain equity or debt financing on acceptable terms or at all to implement our growth strategy. As a result, we cannot assure you that adequate capital will be available to finance our current growth plans, take advantage of business opportunities, or respond to competitive pressures, any of which could harm our business. Additionally, if adequate additional financing is not available on acceptable terms, we may not be able to continue our business operations. Any additional capital, investment or financing of our business may result in dilution of our stockholders or be on terms and conditions that impair our ability to profitably conduct our business.
We are dependent upon the success of our products and technologies, which may not be successfully developed or commercialized.
While the FDA does not require clinical trials for dietary supplements, we have conducted and may continue to conduct clinical trials with our dietary supplements to promote scientific and consumer awareness. We also expect to conduct clinical trials with our pharmaceutical candidates. A failure of any clinical trial can occur at any stage of testing. The results of initial clinical testing may not necessarily indicate the results that will be obtained from later or more extensive testing. Additionally, any observations made with respect to blinded clinical data are inherently uncertain as we cannot know which set of data come from subjects treated with active versus placebo. Investors are cautioned not to rely on observations coming from blinded data and not to rely on initial clinical trial results as necessarily indicative of results that will be obtained in subsequent clinical trials or clinical practice.
Additionally, our products are subject to a variety of FDA and other applicable regulatory authorities. The extent of regulations applicable to our products, and the approvals or designations our products may receive from regulatory authorities, such as the FDA, are dependent upon the nature and development of our products and how such products are ultimately commercialized and marketed.
| 25 |
A number of different factors could prevent us from developing or commercializing our products on a timely basis, or at all.
We, the FDA, other applicable regulatory authorities, or an institutional review board (“IRB”), may suspend clinical trials of a product at any time for various reasons, including if we or they believe the subjects participating in such trials are being exposed to unacceptable health risks. Among other reasons, adverse side effects of a product on subjects in a clinical trial could result in the FDA or other regulatory authorities suspending or terminating the trial and refusing to approve or allow continued marketing of a particular product for any or all indications or applications of use.
Clinical trials require the enrollment of a sufficient number of subjects who meet certain eligibility criteria. Rates of subject enrollment are affected by many factors, and delays in subject enrollment can result in increased costs and longer development times.
Clinical trials also require the review and oversight of IRBs, which approve and continually review clinical investigations and protect the rights and welfare of human subjects. An inability or delay in obtaining IRB approval could prevent or delay the initiation and completion of clinical trials, and the FDA may decide not to consider any data or information derived from a clinical investigation not subject to initial and continuing IRB review and approval.
Numerous factors could affect the timing, cost, or outcome of our development and commercialization efforts, including the following:
| ● | delays in filing or acceptance of IND applications for our pharmaceutical candidates; | |
| ● | difficulty in securing centers to conduct clinical trials; | |
| ● | conditions imposed on us by the FDA or other regulatory authorities that are applicable to our business regarding the scope or design of our clinical trials or the method or scope of our sales and marketing practices; | |
| ● | problems in engaging IRBs to oversee trials or problems in obtaining or maintaining IRB approval of studies; | |
| ● | difficulty in enrolling subjects in conformity with required protocols or projected timelines; | |
| ● | third-party contractors failing to comply with regulatory requirements or to meet their contractual obligations to us in a timely manner; | |
| ● | our products having unexpected and different chemical and pharmacological properties in humans than in laboratory testing and interacting with human biological systems in unforeseen, ineffective or harmful ways; | |
| ● | the need to suspend or terminate clinical trials if the subjects are being exposed to unacceptable health risks; | |
| ● | insufficient or inadequate supply or quality of our products or other materials necessary to conduct our clinical trials; | |
| ● | our products not having the desired effects or having undesirable side effects or other unexpected characteristics; | |
| ● | the cost of our clinical trials being greater than we anticipate; | |
| ● | negative or inconclusive results from our clinical trials or the clinical trials of others for similar products or inability to generate statistically significant data confirming the efficacy or safety of the product being tested; | |
| ● | interim or preliminary results of our clinical trials may not be indicative of the final results for such clinical trials or other clinical trials; | |
| ● | interim or preliminary results of our clinical trials do not ensure that the final results such clinical trial or other clinical trials will be positive or statistically significant or clinically meaningful; | |
| ● | results of our clinical trials may not be replicated by other clinical trials; | |
| ● | changes in the FDA’s other applicable regulatory authorities’ requirements for testing during the course of testing; | |
| ● | reallocation of our limited financial and other resources to other programs; and | |
| ● | adverse results obtained by other companies developing similar products. |
| 26 |
It is possible that none of the products we may develop will obtain the appropriate regulatory approvals necessary to begin selling them or that any regulatory approval to market a product may be subject to limitations on the indicated uses for which we may market the product. The time required to obtain FDA and other approvals is unpredictable, but often can take years following the commencement of clinical trials, depending upon the complexity of the product. Any analysis we perform of data from clinical activities is subject to confirmation and interpretation by regulatory authorities, which could delay, limit or prevent regulatory approval. Any delay or failure in obtaining required approvals could have a material adverse effect on our ability to generate revenue from the particular product.
We also must comply with clinical trial and post-approval safety and adverse event reporting requirements. Adverse events related to our products must be reported to the FDA in accordance with regulatory timelines based on their severity and expectedness. Failure to make timely safety reports and to establish and maintain related records could result in withdrawal of marketing authorization.
We may also become subject to numerous foreign regulatory requirements governing the conduct of clinical trials, manufacturing and marketing authorization, pricing, and third-party reimbursement. The foreign regulatory approval process includes all of the risks associated with the FDA approval described above as well as risks attributable to the satisfaction of local regulations in foreign jurisdictions. Approval by the FDA does not assure approval by regulatory authorities outside of the United States.
If we fail to comply with FDA regulations our business could suffer.
The manufacture and marketing of pharmaceuticals and dietary supplements are subject to extensive regulation by the FDA and foreign and state regulatory authorities. In the United States, pharmaceutical and dietary supplement companies such as ours must comply with laws and regulations promulgated by the FDA. These laws and regulations require various authorizations prior to a product being marketed in the United States. Manufacturing facilities and practices are also subject to FDA regulations. The FDA regulates the clinical testing, manufacture, labeling, sale, distribution, and promotion of pharmaceuticals and dietary supplements in the United States. Our failure to comply with regulatory requirements, including any future changes to such requirements, could have a material adverse effect on our business, prospects, financial condition, and results of operations.
Even after clearance or approval of a product, we are subject to continuing regulation by the FDA, including the requirements of registering our facilities and listing our products with the FDA. We are subject to reporting regulations. These regulations require us to report to the FDA if any of our products may have caused or contributed to a death or serious injury and such product or a similar product that we market would likely cause or contribute to a death or serious injury. Unless an exemption applies, we must report corrections and removals to the FDA where the correction or removal was initiated to reduce a risk to health posed by the product or to remedy a violation of the Food, Drug, and Cosmetic Act. The FDA also requires that we maintain records of corrections or removals, regardless of whether such corrections and removals are required to be reported to the FDA. In addition, the FDA closely regulates promotion and advertising, and our promotional and advertising activities could come under scrutiny by the FDA.
The FDA also requires that manufacturing be in compliance with its Quality System Regulation, or QSR. The QSR covers the methods and documentation of the design, testing, control, manufacturing, labeling, quality assurance, packaging, storage, and shipping of our products. Our failure to maintain compliance with the QSR requirements could result in the shutdown of, or restrictions on, our manufacturing operations, to the extent we have any, and the recall or seizure of our products, which would have a material adverse effect on our business. In the event that one of our suppliers fails to maintain compliance with our quality requirements, we may have to qualify a new supplier and could experience manufacturing delays as a result.
| 27 |
The FDA has broad enforcement powers. If we violate applicable regulatory requirements, the FDA may bring enforcement actions against us, which could have a material adverse effect on our business, prospects, financial condition, and results of operations. Violations of regulatory requirements, at any stage, including after approval, may result in various adverse consequences, including the delay by a regulatory agency in approving or refusal to approve a product, withdrawal or recall of an approved product from the market, other voluntary agency-initiated action that could delay further development or marketing, as well as the imposition of criminal penalties against the manufacturer and NDA holder.
The extent of FDA regulations applicable to us, and whether our products are ultimately designated as drugs (including active pharmaceutical ingredients) or dietary supplements (including dietary ingredients), will depend upon how our products are ultimately commercialized. Furthermore, our products may be commercialized by us or by other parties through licensing arrangements, joint ventures, or other alliances, and our burden of complying with any regulations applicable to our products will depend upon the nature and extent of any relationships with such partners. While dietary supplements are not as extensively regulated as pharmaceuticals, the extent of any regulations to which we may be subject will depend upon the specific products we ultimately produce.
We have limited experience in managing communications with regulatory authorities, including filing IND applications, filing new drug applications, submitting promotional materials, and generally directing the regulatory processes in all territories.
We may be responsible for managing communications with regulatory authorities, including filing INDs, filing NDAs, submitting promotional materials, and generally directing the regulatory processes in all territories. We have limited experience directing such activities and may not be successful with our planned development strategies, on the planned timelines, or at all. Even if any of our products are designated for “fast track” or “priority review” status or if we seek approval under accelerated approval (Subpart H) regulations, such designation or approval pathway does not necessarily mean a faster development process or regulatory review process or necessarily confer any advantage with respect to approval compared to conventional FDA procedures. Accelerated development and approval procedures will only be available if the indications for which we are developing products remain unmet medical needs and if our clinical trial results support use of surrogate endpoints, respectively. Even if these accelerated development or approval mechanisms are available to us, depending on the results of clinical trials, we may elect to follow the more traditional approval processes for strategic and marketing reasons, since drugs approved under accelerated approval procedures are more likely to be subjected to post-approval requirements for clinical studies to provide confirmatory evidence that the drugs are safe and effective. If we fail to conduct any such required post-approval studies or if the studies fail to verify that any of our products are safe and effective, our FDA approval could be revoked. It can be difficult, time-consuming, and expensive to enroll patients in such clinical trials because physicians and patients are less likely to participate in a clinical trial to receive a drug that is already commercially available. Drugs approved under accelerated approval procedures also require regulatory pre-approval of promotional materials that may delay or otherwise hinder commercialization efforts.
We expect to continue to incur significant research and development expenses, which may make it difficult for us to attain profitability.
We expend substantial funds to develop our products, and additional substantial funds will be required for further research and development, including preclinical and clinical testing, and to manufacture and market any products that are approved for commercial sale. Because the successful development of our products is uncertain, we are unable to precisely estimate the actual funds we will require to develop and potentially commercialize them. In addition, we may not be able to generate enough revenue, even if we are able to commercialize any of our products, to become profitable.
| 28 |
We operate in highly competitive industries, and our failure to compete effectively could adversely affect our market share, financial condition and growth prospects. If competitors are better able to develop and market products that are more effective, or gain greater acceptance in the marketplace than our products, our commercial opportunities may be reduced or eliminated.
The dietary supplement and pharmaceutical industries are constantly evolving, and scientific advances are expected to continue at a rapid pace. This results in intense competition among companies operating in the industry. Other, larger companies may have, or may be developing, products that compete with our products and may significantly limit the market acceptance of our products or render them obsolete. Our technical and/or business competitors would include major pharmaceutical companies, biotechnology companies, consumer health companies, universities, and nonprofit research institutions and foundations. Most of these competitors have significantly greater research and development capabilities than we have, as well as substantial marketing, financial, and managerial resources. ZanthoSyn®, our lead product, primarily competes against products that provide anti-inflammatory health benefits. In addition, there are several other companies, both public and private, that service the same markets as we do, all of which compete to some degree with us.
The primary competitive factors facing us include safety, efficacy, price, quality, breadth of product line, manufacturing quality and capacity, service, marketing, and distribution capabilities. Our current and future competitors may have greater resources, more widely accepted and innovative products and stronger name recognition than we do. Our ability to compete is affected by our ability to:
| ● | develop or acquire new products and innovative technologies; | |
| ● | obtain regulatory clearance and compliance for our products; | |
| ● | manufacture and sell our products cost-effectively; | |
| ● | meet all relevant quality standards for our products in their particular markets; | |
| ● | respond to competitive pressures specific to each of our geographic and product markets; | |
| ● | protect the proprietary technology of our products and avoid infringement of the proprietary rights of others; | |
| ● | market our products; | |
| ● | attract and retain skilled employees, including sales representatives; | |
| ● | maintain and establish distribution relationships; and | |
| ● | engage in acquisitions, joint ventures, or other collaborations. |
Competitors could develop products that are more effective, achieve favorable reimbursement status from third-party payors, cost less, or are ready for commercial introduction before our products. If our competitors are better able to develop and patent products earlier than we can, or develop more effective and/or less expensive products that render our products obsolete or non-competitive, our business will be harmed and our commercial opportunities will be reduced or eliminated.
In addition, competitors and other parties may also seek to impact regulatory status of our products through the filing of citizen petitions or other similar documents.
We believe that the market in which we compete in is also highly sensitive to the introduction of new products, including various prescription drugs, which may rapidly capture a significant share of the market. In the United States, we expect to also compete for sales with heavily advertised national brands manufactured by large pharmaceutical, biotechnology, and consumer health companies, as well as other retailers.
As some products gain market acceptance, we may experience increased competition for those products as more participants enter the market. Currently, we are not a manufacturer. To the extent that we engage third-party manufacturers or use strategic alliances to produce our products, our manufacturing capabilities may not be adequate or sufficient to compete with large scale, direct, or third-party manufacturers. Certain of our potential competitors are larger than us and have longer operating histories, customer bases, greater brand recognition, and greater resources for marketing, advertising, and product promotion. They may be able to secure inventory from vendors on more favorable terms, operate with a lower cost structure, or adopt more aggressive pricing policies. In addition, our potential competitors may be more effective and efficient in introducing new products. We may not be able to compete effectively, and our attempt to do so may require us to increase marketing and/or reduce our prices, which may result in lower margins. Failure to effectively compete could adversely affect our market share, financial condition, and growth prospects.
| 29 |
The pharmaceutical and dietary supplement industries are subject to extensive and complex healthcare regulation. Any determination that we have violated federal or state laws applicable to us that regulate healthcare would have a material adverse effect on our business, prospects, and financial condition.
Federal and state laws regulating healthcare are extensive and complex. The laws applicable to our business are subject to evolving interpretations, and therefore we cannot be sure that a review of our operations by federal or state courts or regulatory authorities will not result in a determination that we have violated one or more provisions of federal or state law. Any such determination could have a material adverse effect on our business, prospects, and financial condition.
Healthcare and insurance legislation may increase the difficulty and cost for us to commercialize our products and affect the prices we may obtain.
The United States and many foreign jurisdictions have enacted or proposed legislative and regulatory changes affecting the healthcare system that could prevent or delay marketing approval of our products, restrict or regulate post-approval activities, and affect our ability to profitably sell any product for which we obtain marketing approval.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or Medicare Modernization Act, changed the way Medicare covers and pays for pharmaceuticals. The legislation expanded Medicare coverage for drug purchases by the elderly by establishing Medicare Part D and introduced a new reimbursement methodology based on average sales prices for physician-administered drugs under Medicare Part B. In addition, this legislation provided authority for limiting the number of drugs that Medicare will cover in any therapeutic class under the new Medicare Part D program. Cost reduction initiatives and other provisions of this legislation could decrease the coverage and reimbursement rate that we receive for any of our approved products. While the Medicare Modernization Act applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the Medicare Modernization Act may result in a similar reduction in payments from private payors.
In March 2010, former President Obama signed into law the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or, collectively, the Affordable Care Act, a law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against healthcare fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on pharmaceutical and medical device manufacturers, and impose additional health policy reforms. Among other things, the Affordable Care Act expanded manufacturers’ rebate liability under the Medicaid Drug Rebate Program by increasing the minimum rebate for both branded and generic drugs, effective the first quarter of 2010, and revising the definition of “average manufacturer price,” or AMP, for reporting purposes, which could increase the amount of Medicaid drug rebates manufacturers are required to pay to states. The legislation also extended Medicaid drug rebates, previously due only on fee-for-service utilization, to Medicaid managed care utilization, and created an alternative rebate formula for certain new formulations of certain existing products that is intended to increase the amount of rebates due on those drugs.
The Centers for Medicare and Medicaid Services, which administers the Medicaid Drug Rebate Program, also has proposed to expand Medicaid drug rebates to the utilization that occurs in the United States territories, such as Puerto Rico and the Virgin Islands. Also effective in 2010, the Affordable Care Act expanded the types of entities eligible to receive discounted 340B pricing, although, with the exception of children’s hospitals, these newly eligible entities will not be eligible to receive discounted 340B pricing on orphan drugs. In addition, because 340B pricing is determined based on AMP and Medicaid drug rebate data, the revisions to the Medicaid rebate formula and AMP definition described above could cause the required 340B discounts to increase. Furthermore, as of 2011, the new law imposes a significant annual fee on companies that manufacture or import branded prescription drugs and requires manufacturers to provide a 50% discount off the negotiated price of prescriptions filled by beneficiaries in the Medicare Part D coverage gap, referred to as the “donut hole.” Substantial new provisions affecting compliance have also been enacted, which may affect our business practices with healthcare practitioners. Notably, a significant number of provisions are not yet, or have only recently become, effective. Although it is too early to determine the full effect of the Affordable Care Act, the new law appears likely to continue the downward pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
| 30 |
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. In August 2011, the former President signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee on Deficit Reduction did not achieve a targeted deficit reduction of at least $1.2 trillion for fiscal years 2012 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers of up to 2% per fiscal year.
We expect that the Affordable Care Act, as well as other healthcare reform measures that have and may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product, and could seriously harm our future revenues. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our products.
The impact of continued health care reform efforts with respect to the Affordable Care Act is currently unknown, and may adversely affect our business model.
Since its enactment, there have been judicial and Congressional challenges to numerous provisions of the Affordable Care Act. In January 2017, Congress voted to adopt a budget resolution for fiscal year 2017, or the Budget Resolution, that authorizes the implementation of legislation that would repeal portions of the Affordable Care Act. The Budget Resolution is not a law, but it is widely viewed as the first step toward the passage of legislation that would repeal certain aspects of the Affordable Care Act. On January 20, 2017, President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under the Affordable Care Act to waive, defer, grant exemptions from, or delay the implementation of any provision of the Affordable Care Act that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. Additionally, on October 12, 2017, President Trump issued another executive order requiring the Secretaries of the Departments of Health and Human Services (“HHS”), Labor, and the Treasury to consider proposing regulations or revising existing guidance to allow more employers to form association health plans that would be allowed to provide coverage across state lines, increase the availability of short-term, limited duration health insurance plans, which are generally not subject to the requirements of the Affordable Care Act, and increase the availability and permitted use of health reimbursement arrangements. On October 13, 2017, the Department of Justice announced that HHS was immediately stopping its cost sharing reduction payments to insurance companies based on the determination that those payments had not been appropriated by Congress. Furthermore, on December 22, 2017, President Trump signed tax reform legislation into law that, in addition to overhauling the federal tax system, also, effective as of January 1, 2019, repeals the penalties associated with the individual mandate. Congress or the President of the United States may also consider subsequent legislation or executive action to replace or eliminate elements of the Affordable Care Act. We will continue to evaluate the effect that the Affordable Care Act and any future measures to modify, repeal or replace the Affordable Care Act have on our business. We are not able to provide any assurance that the continued healthcare reform debate will not result in legislation, regulation, or executive action by the President of the United States that is adverse to our business. We expect continued development in health care reform and cannot provide any assurance that any changes will not be adverse to us our products or strategies.
US trade policy could adversely affect our costs.
The future of U.S. trade policies is not certain and may have an effect on the global economy including our ability or our third-party manufacturers’ ability to source product or components from certain countries. The response to current U.S. trade policy by sovereign nations is dynamic and cannot be predicted by us. Other nations may reciprocate in trade tariffs or take other actions that could have an adverse effect on the U.S. economy in general and our ability or our third-party manufacturers’ ability to acquire raw materials or inventory at acceptable prices.
| 31 |
Orphan drug designation for our products may not confer marketing exclusivity or other expected benefits.
Under the Orphan Drug Act of 1983 (the “Orphan Drug Act”), the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition that (i) affects less than 200,000 persons in the United States, or (ii) affects more than 200,000 in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for such disease or condition will be recovered from sales in the United States of such drug. The Orphan Drug Act mainly provides incentives intended to make the development of orphan drugs financially viable but does not provide for separate regulatory standards for orphan drugs. Drugs that receive an orphan drug designation do not require prescription drug user fees at the time of marketing application, may qualify the drug development sponsor for certain tax credits, and can be marketed without generic competition for seven years.
We are seeking orphan drug designation for certain products that we believe may qualify for orphan drug designation; however, there can be no assurance that we will request an orphan drug designation for any product, or if requested, that we will receive such orphan drug designation. If we are unable to secure orphan drug designation, our regulatory and commercial prospects may be negatively impacted. Even if we obtain orphan drug designation for a product, we may not be able to obtain marketing approval or maintain orphan drug exclusivity for that product. We may not be the first to obtain marketing approval of any product for which we have obtained orphan drug designation for the orphan-designated indication due to the uncertainties associated with developing pharmaceuticals. In addition, exclusive marketing rights in the United States may be limited if we seek approval for an indication broader than the orphan-designated indication or may be lost if the FDA later determines that the request for designation was materially defective or if we are unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition. Further, even if we obtain orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different drugs with different active moieties may be approved for the same condition. Even after an orphan drug is approved, the FDA can subsequently approve the same drug with the same active moiety for the same condition if the FDA concludes that the later drug is clinically superior in that it is shown to be safer, more effective, or makes a major contribution to patient care, or the manufacturer of the product with orphan exclusivity is unable to maintain sufficient product quantity. Orphan drug designation may not shorten the development time or regulatory review time of a drug or give the drug any advantage in the regulatory review or approval process, nor does it prevent competitors from obtaining approval of the same drug for indications other than those in which we have been granted orphan drug designation.
If we are unable to obtain and maintain protection of our intellectual property, the value of our products may be adversely affected.
Our business is dependent in part upon our ability to use intellectual property rights to protect our products from competition. To protect our products, we rely on a combination of patent and other intellectual property laws, employment, confidentiality, and invention assignment agreements with our employees and contractors, and confidentiality agreements and protective contractual provisions with our partners, licensors, and other third parties. These methods, however, afford us only limited protection against competition from other products.
We attempt to protect our intellectual property position, in part, by filing patent applications and obtaining patents related to our proprietary technology, inventions, and improvements that are important to our business. However, our patent position is not likely by itself to prevent others from commercializing products that compete directly with our products. Moreover, we do not have patent protection for certain components of our products and our patent applications can be challenged. In addition, we may fail to receive any patent for which we have applied, and any patent owned by us or issued to us could be challenged, invalidated, or held to be unenforceable. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability of a patent, we would lose at least part, and perhaps all, of the patent protection on a product. Even if a defendant does not prevail on a legal assertion of invalidity and/or unenforceability, our patent claims may be construed in a manner that would limit our ability to enforce such claims against the defendant and others.
We also note that any patent granted may not provide a competitive advantage to us. Our competitors may independently develop technologies that are substantially similar or superior to our technologies. Further, third parties may design around our patented or proprietary products and technologies.
We rely on certain trade secrets and we may not be able to adequately protect our trade secrets even with contracts with our personnel and third parties. Also, any third party could independently develop and have the right to use, our trade secret, know-how, and other proprietary information. If we are unable to protect our intellectual property rights, our business, prospects, financial condition, and results of operations could suffer materially.
| 32 |
Our ability to market our products may be impaired by the intellectual property rights of third parties.
Our success depends in part on our products not infringing on the patents and proprietary rights of other parties. For instance, in the United States, patent applications filed in recent years are confidential for 18 months, while older applications are not published until the patent issues. As a result, there may be patents and patent applications of which we are unaware, and avoiding patent infringement may be difficult.
Our industry is characterized by a large number of patents, patent applications, and frequent litigation based on allegations of patent infringement. Competitors may own patents or proprietary rights, or have filed patent applications, related to products that are similar to ours. We may not be aware of all of the patents and pending applications potentially adverse to our interests that may have been issued to others. Moreover, since there may be unpublished patent applications that could result in patents with claims relating to our products, we cannot be sure that our current products will not infringe any patents that might be issued or filed in the future. Based on the litigious nature of our industry and the fact that we may pose a competitive threat to some companies who own or control various patents, we believe it is possible that one or more third parties may assert a patent infringement claim seeking damages or enjoining us from the manufacture or marketing of one or more of our products. Such a lawsuit may have already been filed against us without our knowledge or may be filed in the future. If any future claim of infringement against us was successful, we may be required to pay substantial damages, cease the infringing activity, or obtain the requisite licenses or rights to use the technology, which may not be available to us on acceptable terms, if at all. Even if we were able to obtain rights to a third party’s intellectual property rights, these rights may be non-exclusive, thereby giving our competitors potential access to the same rights and weakening our market position. Moreover, regardless of the outcome, patent litigation could significantly disrupt our business, divert our management’s attention and consume our financial resources. We cannot predict if or when any third-party patent holder will file suit for patent infringement.
We may be involved in lawsuits or proceedings to protect or enforce our intellectual property rights or to defend against infringement claims, which could be expensive and time consuming.
Litigation may be necessary to enforce our intellectual property rights, protect our trade secrets, or determine the validity and scope of the proprietary rights of others. Interference proceedings conducted by a patent and trademark office may be necessary to determine the priority of inventions with respect to our patent applications. Litigation or interference proceedings, including the defense against infringement or invalidity claims, would be expensive and could result in substantial costs and diversion of resources and management attention. In addition, in an infringement proceeding, a court may decide that a patent of ours is not valid or is unenforceable or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology or the product. An adverse determination of any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing. In addition, we may be enjoined from marketing one or more of our products if a court finds that such products infringe the intellectual property rights of a third party.
During litigation, we may not be able to prevent the confidentiality of certain of our proprietary rights because of the substantial amount of discovery required in connection with intellectual property litigation. In addition, during the course of litigation, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments. If investors or customers perceive these results to be negative, it could have a material adverse effect on our business, prospects, financial condition, and results of operations.
Commercialization of our products requires sophisticated sales and marketing teams.
We have limited prior experience with commercializing our products. To successfully continue to commercialize our dietary supplement products and to commercialize any pharmaceutical products, we need to establish and maintain sophisticated sales and marketing teams and/or utilize the resources of any licensee, contractor, or other third party. While we intend to use current Company employees and service providers to lead our marketing efforts, we may choose to expand our marketing and sales team. Experienced sales representatives may be difficult to locate and retain, and all new sales representatives will need to undergo extensive training. There is no assurance that we will be able to recruit and retain sufficiently skilled sales representatives, or that any new sales representatives will ultimately become productive. If we are unable to recruit and retain qualified and productive sales personnel, our ability to commercialize our products and to generate revenues will be impaired, and our business will be harmed.
| 33 |
We have limited experience as a commercial company.
In 2016, we launched our first commercial product, ZanthoSyn®, and we have limited sales to date. As such, we have limited historical financial data upon which to base our projected revenue, planned operating expenses or upon which to evaluate our company and our commercial prospects. Based on our limited experience in developing and marketing new products, we may not be able to effectively:
| ● | drive adoption of our current and future products, including ZanthoSyn®; | |
| ● | attract and retain customers for our products; | |
| ● | provide appropriate levels of customer support for our products; | |
| ● | implement effective marketing strategies to promote awareness of our products; | |
| ● | develop, manufacture, and commercialize new products or achieve an acceptable return on our research and development efforts and expenses; | |
| ● | comply with regulatory requirements applicable to our products; | |
| ● | anticipate and adapt to changes in our market; | |
| ● | maintain and develop strategic relationships with vendors and manufacturers to acquire necessary materials for the production of our existing or future products; | |
| ● | scale our manufacturing activities to meet potential demand at a reasonable cost; | |
| ● | avoid infringement and misappropriation of third-party intellectual property; | |
| ● | obtain any necessary licenses to third-party intellectual property on commercially reasonable terms; | |
| ● | obtain valid and enforceable patents that give us a competitive advantage; | |
| ● | protect our proprietary technology; and | |
| ● | attract, retain, and motivate qualified personnel. |
In addition, a high percentage of our expenses is and will continue to be fixed. Accordingly, if we do not generate revenue as and when anticipated, our losses may be greater than expected and our operating results will suffer.
Market acceptance of ZanthoSyn® and any future products are vital to our future success.
The commercial success of ZanthoSyn® and any future products is dependent upon the acceptance of such products. ZanthoSyn® and any future products may not gain and maintain any significant degree of market acceptance among potential consumers, retailers, healthcare providers, or acceptance by third-party payors, such as health insurance companies. The health applications for ZanthoSyn® and any future products can also be addressed by other products or techniques. The medical community widely accepts alternative treatments, and certain of these other treatments have a long history of use. We cannot be certain that our proposed products and the procedures in which they are used will be able to replace those established treatments or that users will accept and utilize our products or any other medical products that we may market.
Market acceptance will depend upon numerous factors, many of which are not under our control, including:
| ● | the safety and efficacy of our products; | |
| ● | favorable regulatory approval and product labeling; | |
| ● | the availability, safety, efficacy, and ease of use of alternative products or treatments; | |
| ● | our ability to educate potential users on the advantages of our products; | |
| ● | the price of our products relative to alternative technologies; | |
| ● | the availability of third-party reimbursement; and | |
| ● | our distribution channels and any support by retailers. |
If our proposed products do not achieve significant market acceptance, our future revenues and profitability would be adversely affected.
| 34 |
We have limited experience in marketing our products.
We have undertaken limited marketing efforts for ZanthoSyn® and any future products. Our sales and marketing teams compete against the experienced and well-funded sales organizations of competitors. Our future revenues and ability to achieve profitability will depend largely on the effectiveness of our sales and marketing team, and we will face significant challenges and risks related to marketing our services, including, but not limited to, the following:
| ● | the ability of sales representatives to obtain access to or persuade adequate numbers of healthcare providers to recommend and/or purchase and/or use our products; | |
| ● | the ability to recruit, properly motivate, retain, and train adequate numbers of qualified sales and marketing personnel; | |
| ● | the costs associated with hiring, training, maintaining, and expanding an effective sales and marketing team; and | |
| ● | assuring compliance with government regulatory requirements affecting the healthcare industry in general and our products in particular. |
We may seek to establish a network of distributors in selected markets to market, sell, and distribute our products. If we fail to select or use appropriate distributors, or if the sales and marketing strategies of such distributors prove ineffective in generating sales of our products, our future revenues would be adversely affected, and we might never become profitable.
The loss of our largest customer would substantially reduce revenues.
Our customers are material to our success. If we are unable to maintain good relationships with our existing customers, our business could suffer. We sell ZanthoSyn® to GNC under a purchasing agreement. The exclusivity provision under such agreement related to distribution of ZanthoSyn® by GNC in the “brick and mortar” retail channel in the United States expired on October 16, 2019. GNC remains our only distributor of ZanthoSyn® in such channel, but we may expand retail distribution to mass market retailers, other specialty nutrition stores, pharmacies, and other retailers. We cannot provide assurance that GNC will continue to sell ZanthoSyn® at the same levels, or at all.
Commercialization of our products requires sophisticated sales and marketing teams.
We have limited prior experience with commercializing our products. To successfully continue to commercialize our dietary supplement products and to commercialize any pharmaceutical products, we need to establish and maintain sophisticated sales and marketing teams and/or utilize the resources of any licensee, contractor, or other third party. While we intend to use current Company employees and service providers to lead our marketing efforts, we may choose to expand our marketing and sales team. Experienced sales representatives may be difficult to locate and retain, and all new sales representatives will need to undergo extensive training. There is no assurance that we will be able to recruit and retain sufficiently skilled sales representatives, or that any new sales representatives will ultimately become productive. If we are unable to recruit and retain qualified and productive sales personnel, our ability to commercialize our products and to generate revenues will be impaired, and our business will be harmed.
An unexpected interruption or shortage in the supply or significant increase in the cost of components could limit our ability to manufacture any products, which could reduce our sales and margins.
To the extent we engage in relationships with contract manufacturers in the future, an unexpected interruption of supply or a significant increase in the cost of components, whether to us or to our contract manufacturers for any reason, such as regulatory requirements, import restrictions, loss of certifications, disruption of distribution channels as a result of weather, terrorism or acts of war, or other events, could result in significant cost increases and/or shortages of our products. Our inability to obtain sufficient amounts of our products or to pass through higher cost of products we offer could have a material adverse effect on our business, financial condition, or results of operations.
| 35 |
We rely on third parties to supply and manufacture our products. If these third parties do not perform as expected or if our agreements with them are terminated, our business, prospects, financial condition, and results of operations would be materially adversely affected.
We outsource our manufacturing to third parties. Our reliance on contract manufacturers and suppliers exposes us to risks, including the following:
| ● | We rely on our suppliers and manufacturers to provide us with the needed products or components in a timely fashion and of an acceptable quality. An uncorrected defect or supplier’s variation in a component could harm our or our third-party manufacturers’ ability to manufacture, and our ability to sell, products and may subject us to product liability claims. | |
| ● | The facilities of our third-party manufacturers must satisfy production and quality standards set by applicable regulatory authorities. Regulatory authorities periodically inspect manufacturing facilities to determine compliance with these standards. If we or our third-party manufacturers fail to satisfy these requirements, the facilities could be shut down. | |
| ● | These manufacturing operations could also be disrupted or delayed by fire, earthquake or other natural disaster, a work stoppage or other labor-related disruption, failure in supply or other logistical channels, electrical outages, or other reasons. If there was any such disruption to any of these manufacturing facilities, our third-party manufacturers would potentially be unable to manufacture our products. | |
| ● | A third-party manufacturer or supplier could decide to terminate our manufacturing or supply arrangement, including due to a disagreement between us and such third-party manufacturer, if the third-party manufacturer determines not to further manufacture our products, or if we fail to comply with our obligations under such arrangements. | |
| ● | If any third-party manufacturer makes improvements in the manufacturing process for our products, we may not own, or may have to share, the intellectual property rights to the innovation. |
We currently rely on a limited number of suppliers to provide key components for our products. If these or other suppliers become unable to provide components in the volumes needed or at an acceptable price or quality, we would have to identify and qualify acceptable replacements from alternative suppliers. We may experience stoppages in the future. We may not be able to find a sufficient alternative supplier in a reasonable time period, or on commercially reasonable terms, if at all, and our ability to produce and supply our products could be impaired.
To the extent we are able to identify alternative suppliers, qualifying suppliers is a lengthy process. There are a limited number of manufacturers and suppliers that may satisfy applicable requirements. In addition, FDA regulations may require additional testing of any components from new suppliers prior to our use of these materials or components, which testing could delay or prevent the supply of components. Moreover, a new manufacturer would have to be educated in, or develop substantially equivalent processes for, production of our products, which could take a significant period of time.
Each of these risks could delay the development or commercialization of our products or result in higher costs or deprive us of potential product revenues. Furthermore, delays or interruptions in the manufacturing process could limit or curtail our ability to meet demand for our products and/or make commercial sales, unless and until the manufacturing capability at the facilities are restored and re-qualified or alternative manufacturing facilities are developed or brought on-line and “scaled up.” Any such delay or interruption could have a material adverse effect on our business, prospects, financial condition, and results of operations.
| 36 |
We may rely on third-party distributors for sales, marketing, and distribution activities.
We may rely on third-party distributors to sell, market, and distribute ZanthoSyn® and any future products. Because we may rely on third-party distributors for sales, marketing, and distribution activities, we may be subject to a number of risks associated with our dependence on these third-party distributors, including:
| ● | lack of day-to-day control over the activities of third-party distributors; | |
| ● | third-party distributors may not fulfill their obligations to us or otherwise meet our expectations; | |
| ● | third-party distributors may terminate their arrangements with us on limited or no notice or may change the terms of these arrangements in a manner unfavorable to us for reasons outside of our control; and | |
| ● | disagreements with our distributors could require or result in costly and time-consuming litigation or arbitration. |
If we fail to establish and maintain satisfactory relationships with third-party distributors, we may be unable to sell, market, and distribute our products, our future revenues and market share may not grow as anticipated, and we could be subject to unexpected costs which would harm our results of operations and financial condition. There is no assurance that our sales through retail stores will be on terms that are favorable to us or at all.
We may not be able to establish or maintain the third-party relationships that are necessary to develop or potentially commercialize some or all of our products.
We expect to depend on collaborators, partners, licensees, contract research organizations, contract manufacturing organizations, clinical research organizations, and other third parties to support our discovery efforts, to manufacture our products and to conduct clinical trials for some or all of our products. We cannot guarantee that we will be able to successfully negotiate agreements for or maintain relationships with collaborators, partners, licensees, contractors, clinical investigators, vendors, and other third parties on favorable terms, if at all. Our ability to successfully negotiate such agreements will depend on, among other things, potential partners’ evaluation of the superiority of our technology over competing technologies, the quality of the preclinical and clinical data that we have generated and the perceived risks specific to developing our products. If we are unable to obtain or maintain these agreements, we may not be able to develop, manufacture, obtain regulatory approvals for, or commercialize our products. We cannot necessarily control the amount or timing of resources that our contract partners will devote to our research and development programs, products or potential products, and we cannot guarantee that these parties will fulfill their obligations to us under these arrangements in a timely fashion. We may not be able to readily terminate any such agreements with contract partners even if such contract partners do not fulfill their obligations to us. We may experience stoppages in the future. We may not be able to find a sufficient alternative provider in a reasonable time period, or on commercially reasonable terms, if at all, and our ability to produce and supply our products could be impaired.
We may be subject to product liability claims. Our insurance may not be sufficient to cover these claims, or we may be required to recall our products.
Our business is to develop and commercialize, among other things, pharmaceuticals and dietary supplements. As a result, we will face an inherent risk of product liability claims. The pharmaceutical and dietary supplement industries have been historically litigious. Since our products are to be used in the human body, manufacturing errors, design defects, or packaging defects could result in injury or death to the patient or consumer. This could result in a recall of one or more of our products and substantial monetary damages. Any product liability claim brought against us, with or without merit, could result in a diversion of our resources, an increase in our product liability insurance premiums, and/or an inability to secure coverage in the future. We may also have to pay any amount awarded by a court in excess of our policy limits. In addition, any recall of our products, whether initiated by us or by a regulatory authority, may result in adverse publicity for us that could have a material adverse effect on our business, prospects, financial condition, and results of operations. Our product liability insurance policies have various exclusions; therefore, we may be subject to a product liability claim or recall for which we have no insurance coverage. In such a case, we may have to pay the entire amount of the award or costs of the recall. Finally, product liability insurance supplements or renewals may be expensive and may not be available in the future on acceptable terms, or at all.
| 37 |
If we experience product recalls, we may incur significant and unexpected costs and damage to our reputation and, therefore, could have a material adverse effect on our business, financial condition, or results of operations.
We may be subject to product recalls, withdrawals, or seizures if any of our products are believed to cause injury or illness or if we are alleged to have violated governmental regulations in the manufacture, labeling, promotion, sale, or distribution of our products. A recall, withdrawal, or seizure of any of our products could materially and adversely affect consumer confidence in our brands and lead to decreased demand for our products. In addition, a recall, withdrawal, or seizure of any of our products would require significant management attention, would likely result in substantial and unexpected expenditures and could materially and adversely affect our business, financial condition, or results of operations.
Our insurance liability coverage is limited and may not be adequate to cover potential losses.
In the ordinary course of business, we purchase insurance coverage (e.g., liability coverage) to protect us against claims made by third parties and employees for product liability, property damage, or personal injuries. However, the protection provided by such insurance is limited in significant respects and, in some instances, we have no coverage and certain of our insurance policies have substantial “deductibles” or have limits on the maximum amounts that may be recovered. Insurers also have exclusions or limitations of coverage for claims related to certain perils including, but not limited to, product liability, mold, and terrorism. If a series of losses occurred, such as from a series of lawsuits, each of which were subject to the deductible amount, or if the maximum limit of the available insurance was substantially exceeded, we could incur losses in amounts that would have a material adverse effect on our results of operations and financial condition.
Our operating results may fluctuate, which may result in volatility of our share price.
Our operating results, including components of operating results, can be expected to fluctuate from time to time in the future. Some of the factors that may cause these fluctuations include:
| ● | the impact of acquisitions; | |
| ● | market acceptance of our existing products, as well as products in development; | |
| ● | the timing of regulatory approvals; | |
| ● | our ability or the ability of third-party distributors to sell, market, and distribute our products; | |
| ● | our ability or the ability of our contract manufacturers to manufacture our products efficiently; and | |
| ● | the timing of our research and development expenditures. |
If we are unable to manage our expected growth, our future revenue and operating results may be adversely affected.
Our anticipated growth is expected to place a significant strain on our management, operational and financial resources. Our current and planned personnel, systems, procedures and controls may not be adequate to support our anticipated growth. To manage our growth, we will be required to improve existing, and implement new, operational and financial systems, procedures, and controls, and to expand, train, and manage our growing employee base. We expect that we may need to increase our management personnel to oversee our expanding operations. Recruiting and retaining qualified individuals can be difficult. If we are unable to manage our growth effectively, or are unsuccessful in recruiting qualified management personnel, our business, prospects, financial condition, and results of operations could be harmed.
You may have limited access to information regarding our Company because we are a limited reporting company exempt from many regulatory requirements.
As a filer subject to Section 15(d) of the Exchange Act, the Company is not required to prepare proxy or information statements; our common stock is not subject to the protection of the going private regulations; the Company is subject to only limited portions of the tender offer rules; our officers, directors, and more than ten (10%) percent stockholders are not required to file beneficial ownership reports about their holdings in our Company; such persons are not subject to the short-swing profit recovery provisions of the Exchange Act; and stockholders of more than five percent (5%) are not required to report information about their ownership positions in the securities. As a result, investors will have reduced visibility as to the Company and its financial condition.
| 38 |
Risks Related to Ownership of Our Common Stock
Our common stock has a limited trading market, which could affect your ability to sell shares of our common stock and the price you may receive for our common stock.
Our common stock is currently traded in the over-the-counter market and “bid” and “asked” quotations regularly appear on the OTCQB maintained by OTC Markets, Inc. under the symbol “CDXI”. There is only limited trading activity in our securities. We have a relatively small public float compared to the number of our shares outstanding. Accordingly, we cannot predict the extent to which investors’ interest in our common stock will provide an active and liquid trading market, which could depress the trading price of our common stock and could have a long-term adverse impact on our ability to raise capital in the future. Due to our limited public float, we may be vulnerable to investors taking a “short position” in our common stock, which would likely have a depressing effect on the price of our common stock and add increased volatility to our trading market. The volatility of the market for our common stock could have a material adverse effect on our business, results of operations, and financial condition. There cannot be any guarantee that an active trading market for our securities will develop or, if such a market does develop, will be sustained. Accordingly, investors must be able to bear the financial risk of losing their entire investment in our common stock.
After giving effect to the Reverse Stock Split, the number of shares of our common stock outstanding, including the number of shares in the public float, was substantially reduced, which decreased the liquidity of our common stock and may increase the volatility of our common stock. Furthermore, the increase in the market price of our common stock as a result of the Reverse Stock Split has not increased our market value given the reduced number of shares outstanding.
We may voluntarily file for deregistration of our common stock with the Commission.
Compliance with the periodic reporting requirements required by the Securities and Exchange Commission (the “Commission” or “SEC”) consumes a considerable amount of both internal, as well external, resources and represents a significant cost for us. Our senior management team has relatively limited experience managing a company subject to the reporting requirements of the Exchange Act, and the regulations promulgated thereunder. Our management will be required to design and implement appropriate programs and policies in responding to increased legal, regulatory compliance, and reporting requirements, and any failure to do so could lead to the imposition of fines and penalties and harm our business. In addition, if we are unable to continue to devote adequate funding and the resources needed to maintain such compliance, while continuing our operations, we may be in non-compliance with applicable SEC rules or the securities laws, and be delisted from the OTCQB or other market we may be listed on, which would result in a decrease in or absence of liquidity in our common stock, and potentially subject us and our officers and directors to civil, criminal, and/or administrative proceedings and cause us to voluntarily file for deregistration of our common stock with the Commission.
Future sales of our common stock in the public market could lower the price of our common stock and impair our ability to raise funds in future securities offerings.
We intend to raise additional capital through the sale of our securities. Future sales of a substantial number of shares of our common stock in the public market, or the perception that such sales may occur, could adversely affect the then prevailing market price of our common stock and could make it more difficult for us to raise funds in the future through the sale of our securities.
Future sales of our common stock may cause significant dilution to our stockholders.
We intend to raise additional capital through the sale of our securities. If we raise additional funds by issuing shares of our common stock or securities convertible into our common stock, we will reduce the percentage ownership of our then existing stockholders. If the Proposed Public Offering is consummated, our then existing stockholders will be diluted significantly.
We have a substantial number of outstanding options, warrants, and other convertible securities, which may cause significant dilution to our stockholders.
We have a substantial number of outstanding options, warrants, and convertible notes, which may cause significant dilution to our stockholders upon exercise or conversion into shares of our common stock. These options, warrants, and convertible securities provide the right to purchase shares of our common stock at a price that may be less than the then prevailing market price per share of our common stock. In addition, certain of these securities include anti-dilution or repricing provisions that reduce the exercise or conversion price per share of our common stock if we issue shares of our common stock at a price that is lower than the then current exercise or conversion price.
| 39 |
The market price of our common stock may be volatile and may be affected by market conditions beyond our control, and you may not be able to sell our securities.
Companies trading in the stock market in general have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our securities, regardless of our actual operating performance.
The market for our common shares is characterized by significant price volatility when compared to seasoned issuers, and we expect that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. The volatility in our share price is attributable to a number of factors. First, our shares of common stock are sporadically and thinly traded. As a consequence of this lack of liquidity, the trading of relatively small quantities of shares by our stockholders may disproportionately influence the price of those shares in either direction. The price for our shares could, for example, decline precipitously in the event that a large number of shares of our common stock are sold on the market without commensurate demand, as compared to a seasoned issuer which could better absorb those sales without adverse impact on its share price. Second, we are a speculative or “risky” investment due to our limited operating history and lack of profits to date, and uncertainty of future market acceptance for our potential products. As a consequence of this enhanced risk, more risk-averse investors may, under the fear of losing all or most of their investment in the event of negative news or lack of progress, be more inclined to sell their shares on the market more quickly and at greater discounts than would be the case with the stock of a seasoned issuer. Many of these factors are beyond our control and may decrease the market price of our common stock, regardless of our operating performance. We cannot make any predictions or projections as to what the prevailing market price for our common stock will be at any time, including as to whether our common stock will sustain its current market price, or as to what effect the sale of shares or the availability of common stock for sale at any time will have on the prevailing market price.
The market price of our common stock is subject to significant fluctuations in response to, among other factors:
| ● | The significant downward pressure on our common stock price caused by the sale of a significant number of shares of our common stock or securities convertible into our common stock, or the issuance of shares of our common stock upon the exercise or conversion of outstanding options, warrants, or other convertible securities, could cause our common stock price to decline, thus allowing short sellers of our common stock an opportunity to take advantage of any decrease in the value of our common stock; | |
| ● | The presence and action of short sellers in our common stock; | |
| ● | market acceptance of our existing products, as well as products in development; | |
| ● | the timing of regulatory approvals; | |
| ● | our ability or the ability of third-party distributors to sell, market, and distribute our products; | |
| ● | our ability or the ability of our contract manufacturers to manufacture our products efficiently; | |
| ● | changes in our financial performance or a change in financial estimates or recommendations by securities analysts; | |
| ● | our ability to raise additional funds to complete development of our pharmaceutical product candidates; | |
| ● | announcements of innovations or new products or services by us or our competitors; | |
| ● | the emergence of new competitors or success of our existing competitors; | |
| ● | operating and market price performance of other companies that investors deem comparable; | |
| ● | sales or purchases of our common stock by insiders; | |
| ● | commencement of, or involvement in, litigation; | |
| ● | changes in governmental regulations; and | |
| ● | general economic conditions and slow or negative growth of related markets. |
In addition, if the market for stock in our industry, or the stock market in general, experiences a loss of investor confidence, the market price of our common stock could decline for reasons unrelated to our business, financial condition or results of operations.
If any of the foregoing occurs, it could cause the price of our common stock to fall and may expose us to lawsuits that, even if unsuccessful, could be costly to defend and distract our Board of Directors and management.
We could be subject to securities class action litigation following a market price decline of our common stock.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because pharmaceutical companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
| 40 |
We may become subject to penny stock regulations and restrictions and if we are subject to such regulations and restrictions you may have difficulty selling shares of our common stock.
The Commission has adopted regulations which generally define so-called “penny stocks” as an equity security that has a market price of less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exemptions. If our common stock becomes a “penny stock”, we will be subject to Rule 15g-9 under the Exchange Act, or the Penny Stock Rule. This rule imposes additional sales practice requirements on broker-dealers that sell such securities to persons other than established customers and “accredited investors” (generally, individuals with a net worth in excess of $1,000,000 or annual income exceeding $200,000, or $300,000 together with their spouses). For transactions covered by Rule 15g-9, a broker-dealer must make a special suitability determination for the purchaser and receive the purchaser’s written consent to the transaction prior to sale. As a result, if our common stock becomes a “penny stock”, this rule may affect the ability of broker-dealers to sell our securities and may affect the ability of purchasers to sell any of our securities in the secondary market.
For any transaction involving a penny stock, unless exempt, the rules require delivery, prior to any transaction in a penny stock, of a disclosure schedule prepared by the Commission relating to the penny stock market. Disclosure is also required to be made about sales commissions payable to both the broker-dealer and the registered representative and current quotations for the securities. Finally, monthly statements are required to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stock.
After giving effect to the Reverse Stock Split, the market price of our common stock increased to more than $5.00 per share and accordingly was no longer a “penny stock”. There can be no assurance that our common stock will continue to not be a “penny stock” because of its price or qualification for exemption from the Penny Stock Rule. In any event, even if our common stock remains exempt from the Penny Stock Rule, we remain subject to Section 15(b)(6) of the Exchange Act, which gives the Commission the authority to restrict any person from participating in a distribution of penny stock if the Commission finds that such a restriction would be in the public interest.
In addition to the “penny stock” rules described above, the Financial Industry Regulatory Authority (“FINRA”) has adopted similar rules that may also limit a stockholder’s ability to buy and sell our common stock. FINRA rules require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for such customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low priced securities will not be suitable for at least some customers. The FINRA requirements may make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our stock and have an adverse effect on the market for our shares.
We do not intend to pay dividends on our common stock so any returns will be limited to the value of our stock.
We have never declared or paid any cash dividends on our common stock or preferred stock. We currently anticipate that we will retain future earnings for the development, operation, and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Any return to common or preferred stockholders will therefore be limited to the appreciation of their stock.
We may issue shares of preferred stock that subordinate your rights and dilute your equity interests.
We believe that for us to successfully execute our business strategy we will need to raise investment capital and it may be preferable or necessary to issue preferred stock to investors. Preferred stock may grant the holders certain preferential rights in voting, dividends, liquidation, or other rights in preference over a company’s common stock.
The issuance by us of preferred stock could dilute both the equity interests and the earnings per share of existing holders of our common stock. Such dilution may be substantial, depending upon the number of shares issued. The newly authorized shares of preferred stock could also have voting rights superior to our common stock, and in such event, would have a dilutive effect on the voting power of our existing stockholders.
Any issuance of preferred stock with voting rights could, under certain circumstances, have the effect of delaying or preventing a change in control of us by increasing the number of outstanding shares entitled to vote and by increasing the number of votes required to approve a change in control of us. Shares of voting or convertible preferred stock could be issued, or rights to purchase such shares could be issued, to render more difficult or discourage an attempt to obtain control of us by means of a tender offer, proxy contest, merger or otherwise. Such issuances could therefore deprive our stockholders of benefits that could result from such an attempt, such as the realization of a premium over the market price that such an attempt could cause. Moreover, the issuance of such shares of preferred stock to persons friendly to our Board of Directors could make it more difficult to remove incumbent managers and directors from office even if such change were to be favorable to stockholders generally.
| 41 |
Provisions in our corporate charter documents and under Delaware law could make an acquisition of us more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our corporate charter and our bylaws may discourage, delay, or prevent a merger, acquisition, or other change in control of us that stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our Board of Directors. Because our Board of Directors is responsible for appointing the members of our management team, these provisions could in turn affect any attempt by our stockholders to replace current members of our management team. Among others, these provisions include the following:
| ● | our Board of Directors will have the right to elect directors to fill a vacancy created by the expansion of our Board of Directors or the resignation, death, or removal of a director, which will prevent stockholders from being able to fill vacancies on our Board of Directors; | |
| ● | our stockholders will not be able to act by written consent or call special stockholders’ meetings; as a result, a holder, or holders, controlling a majority of our capital stock would not be able to take certain actions other than at annual stockholders’ meetings or special stockholders’ meetings called by our Board of Directors, the chairman of our board, the chief executive officer, or the president; | |
| ● | our certificate of incorporation will prohibit cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates; | |
| ● | our stockholders will be required to provide advance notice and additional disclosures in order to nominate individuals for election to our Board of Directors or to propose matters that can be acted upon at a stockholders’ meeting, which may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of our company; and | |
| ● | our Board of Directors will be able to issue, without stockholder approval, shares of undesignated preferred stock, which makes it possible for our Board of Directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to acquire us. |
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
Risks Related to Market Conditions
The sale of material amounts of common stock could encourage short sales by third parties and further depress the price of our common stock. As a result, you may lose all or part of your investment.
The significant downward pressure on our common stock price caused by the sale of a significant number of shares could cause our common stock price to decline, thus allowing short sellers of our common stock an opportunity to take advantage of any decrease in the value of our common stock. The presence of short sellers in our common stock may further depress the price of our common stock.
We have outstanding securities that provide for recognition of derivative liabilities under U.S. Generally Accepted Accounting Principles (“U.S. GAAP”), which may reduce our stockholders’ equity balance.
We have outstanding securities that require us to recognize derivative liabilities under U.S. GAAP as described in the footnotes to our financial statements, including Note 2 and Note 9 to our financial statements for the year ended December 31, 2019. The amount of such derivative liability is determined as of each reporting date and was $827,314 as of December 31, 2019. Because we estimate the fair value of the conversion feature for outstanding securities as a derivative financial instrument at issuance and at each subsequent reporting date using the Black-Scholes valuation model, the amount of the derivative liability may increase during future reporting periods based on the components of such valuation model, which we do not control, and the amount of such derivative liability may be material in future periods. An increase in the derivative liability results in a decrease of our stockholders’ equity. Any such decrease in our stockholders’ equity may adversely impact our ability to satisfy the stockholders’ equity listing requirement for the Nasdaq Capital Market in connection with the Proposed Public Offering.
| 42 |
Natural disasters, public health crises, political crises, and other catastrophic events or other events outside of our control may adversely affect our business.
Natural disasters, public health crises, political crises, and other catastrophic events or other events outside of our control may adversely affect, among other things, our employees, service providers, vendors, suppliers, partners, operations, results of operations, financial condition, and/or market price, which could materially adversely affect our business.
Pandemics including COVID-19 may adversely affect our business.
The recent unprecedented events related to COVID-19, the disease caused by the novel coronavirus (SARS-CoV-2), have had significant health, economic, and market impacts and may have short-term and long-term adverse effects on our business, financial condition, and results of operations that we cannot predict as the global pandemic continues to evolve. The extent and effectiveness of responses by governments and other organizations also cannot be predicted.
We need additional capital to fund our operations and pay our current and future obligations; however, our ability to access the capital markets or otherwise raise such capital is unknown during the COVID-19 pandemic and there can be no assurance that we will be able to obtain sufficient amounts of capital as and when needed. The Proposed Public Offering may be delayed or terminated because of the impacts to the capital markets arising from or related to the COVID-19 pandemic. Any such limitation on available financing would adversely affect our business. Further, the U.S. federal government has responded to the COVID-19 pandemic with economic stimulus programs, but we cannot provide any assurance if these or any other governmental responses or actions will provide any intended economic benefits to us or will improve our access to additional capital in the public or private markets.
Sales of our physician recommended astaxanthin dietary supplement, ZanthoSyn®, especially in the brick-and-mortar retail channel, are uncertain and may decrease as persons stay home in accordance with governmental orders related to COVID-19. ZanthoSyn® is a discretionary consumer healthcare purchase, and as such, our sales may be reduced as consumers focus their cash resources on non-discretionary purchases during the pandemic.
We recently suspended recruitment of new subjects and study visits for existing subjects in the CHASE clinical trial due to the COVID-19 pandemic and the related governmental “stay-at-home” orders. We expect to resume clinical trial operations when permissible and safe to proceed, but we cannot predict the extent of the impact that the pandemic will have on the trial.
Supply or manufacture of ZanthoSyn® or any of our other products or product candidates could be disrupted or delayed as a result of impacts to the global supply chain or to the facilities or personnel of our third-party suppliers or manufacturers arising from or related to the COVID-19 pandemic.
We cannot provide any assurance related to the potential role of astaxanthin as a pharmaceutical in the treatment of COVID-19 or of astaxanthin as a supplement to support immune health or that the white paper we released as described under “Astaxanthin and COVID-19” in this report will be accepted by the scientific community. There are numerous candidates, including vaccines, anti-virals, and anti-inflammatories, being developed or tested for COVID-19 and SARS-CoV-2 by pharmaceutical and biotechnology companies as well as governmental, academic, and other organizations. There can be no assurance that any third-party will pursue a collaboration, joint venture, or other transaction with us to further develop astaxanthin for COVID-19 or immune health, or that astaxanthin will demonstrate any benefits related to COVID-19 or immune health, or that any such potential benefits will lead to any financial or other benefit to us.
Item 1B. Unresolved Staff Comments.
None.
We maintain a facility of approximately 738 square feet at 2800 Woodlawn Drive, Honolulu, Hawaii, which is leased on a month-to-month basis. We believe that our facility is adequate for our current purposes.
From time to time, we may become involved in various lawsuits and legal proceedings that arise in the ordinary course of business. However, litigation is subject to inherent uncertainties and an adverse result in these or other matters may arise from time to time that may harm our business. We are currently not aware of any such legal proceedings or claims that we believe will have a material adverse effect on our business, financial condition or operating results.
Item 4. Mine Safety Disclosures.
Not applicable.
| 43 |
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of EQUITY Securities.
Market Information
Our shares of common stock are quoted on the OTCQB under the symbol “CDXI.” The high and low trading prices for our shares of common stock for each full quarterly period within the two most recent fiscal years are set forth in the table below and have been adjusted for the effect of the Reverse Stock Split, which combined every 200 shares into 1 share as of January 15, 2020:
| Quarter Ended | High | Low | ||||||
| March 31, 2018 | $ | 88.00 | $ | 26.00 | ||||
| June 30, 2018 | $ | 68.00 | $ | 40.00 | ||||
| September 30, 2018 | $ | 48.00 | $ | 34.00 | ||||
| December 31, 2018 | $ | 44.00 | $ | 34.44 | ||||
| March 31, 2019 | $ | 44.00 | $ | 34.00 | ||||
| June 30, 2019 | $ | 40.00 | $ | 14.40 | ||||
| September 30, 2019 | $ | 29.00 | $ | 12.82 | ||||
| December 31, 2019 | $ | 50.00 | $ | 6.40 | ||||
Such quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and do not necessarily represent actual transactions.
Holders
As of March 30, 2020, there were approximately 475 stockholders of record of our common stock. The number of stockholders does not include beneficial owners holding shares through nominee names.
Dividends
We have never paid any cash dividends and intend, for the foreseeable future, to retain any future earnings for the development of our business. Our future dividend policy will be determined by our Board of Directors on the basis of various factors, including our results of operations, financial condition, capital requirements and investment opportunities.
| 44 |
Securities Authorized for Issuance under Equity Compensation Plans
We adopted, and our stockholders approved, the Cardax, Inc. 2014 Equity Compensation Plan, as amended (the “2014 Plan”), effective as of February 7, 2014. Under such plan, we may grant equity-based incentive awards, including options, restricted stock, and other stock-based awards, to any directors, employees, advisors, and consultants that provide services to us or any of our subsidiaries on terms and conditions that are from time to time determined by us. An aggregate of 279,101 shares of our common stock are presently reserved for issuance under the 2014 Plan (the “Plan Shares”). On December 4, 2018, our stockholders and our Board of Directors authorized the annual increase of the Plan Shares on January 1st of each year, at the discretion of our Board of Directors, by up to such number of shares that is equal to four percent (4%) of the shares of our common stock issued and outstanding as of December 31st of the previous calendar year; accordingly, effective as of January 1, 2020, the Plan Shares were increased by 27,000 shares. Options for the purchase of 226,937 shares of our common stock have been granted, options for the purchase of 5,083 shares of our common stock have been exercised, and options for the purchase of 19,608 shares of our common stock have been forfeited; options for the purchase of 202,246 shares of our common stock are outstanding as of the date of this report. In addition, an aggregate of 32,581 shares of our common stock have been granted under the 2014 Plan. The purpose of the 2014 Plan is to provide financial incentives for selected directors, employees, advisors, and consultants of Cardax and/or its subsidiaries, thereby promoting the long-term growth and financial success of the Company.
Equity Compensation Plan Information
The following table summarizes information as of the date of this report about our outstanding stock options and shares of our common stock reserved for future issuance under our existing equity compensation plans.
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants, and rights | Weighted-average exercise price of outstanding options, warrants, and rights | Number of securities remaining available for future issuance under equity compensation plans | |||||||||
| Equity compensation plans approved by security holders | 202,246 | $ | 80.14 | 39,191 | ||||||||
| Equity compensation plans not approved by security holders | - | - | - | |||||||||
| Total | 202,246 | $ | 80.14 | 39,191 | ||||||||
| 45 |
Penny Stock Regulations
The Commission has adopted regulations which generally define so-called “penny stocks” as an equity security that has a market price of less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exemptions. If our common stock becomes a “penny stock”, we will be subject to Rule 15g-9 under the Exchange Act, or the Penny Stock Rule. This rule imposes additional sales practice requirements on broker-dealers that sell such securities to persons other than established customers and “accredited investors” (generally, individuals with a net worth in excess of $1,000,000 or annual income exceeding $200,000, or $300,000 together with their spouses). For transactions covered by Rule 15g-9, a broker-dealer must make a special suitability determination for the purchaser and receive the purchaser’s written consent to the transaction prior to sale. As a result, if our common stock becomes a “penny stock”, this rule may affect the ability of broker-dealers to sell our securities and may affect the ability of purchasers to sell any of our securities in the secondary market.
For any transaction involving a penny stock, unless exempt, the rules require delivery, prior to any transaction in a penny stock, of a disclosure schedule prepared by the Commission relating to the penny stock market. Disclosure is also required to be made about sales commissions payable to both the broker-dealer and the registered representative and current quotations for the securities. Finally, monthly statements are required to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stock.
After giving effect to the Reverse Stock Split, the market price of our common stock increased to more than $5.00 per share and accordingly was no longer a “penny stock”. There can be no assurance that our common stock will continue to not be a “penny stock” because of its price or qualification for exemption from the Penny Stock Rule. In any event, even if our common stock remains exempt from the Penny Stock Rule, we would remain subject to Section 15(b)(6) of the Exchange Act, which gives the Commission the authority to restrict any person from participating in a distribution of penny stock if the Commission finds that such a restriction would be in the public interest.
In addition to the “penny stock” rules described above, the FINRA has adopted similar rules that may also limit a stockholder’s ability to buy and sell our common stock. FINRA rules require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for such customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low priced securities will not be suitable for at least some customers. The FINRA requirements may make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit the ability of our stockholders to sell their shares and have an adverse effect on the market for our shares.
| 46 |
Recent Sales of Unregistered Securities
We issued shares of our common stock or securities convertible into common stock in the following transactions and used the net proceeds for our general working capital and to fund our research, development, and clinical programs.
The securities were issued in reliance upon exemptions from registration pursuant to Section 4(2) of the Securities Act and the rules and regulations promulgated thereunder.
Convertible Notes
We entered into convertible notes payable with lenders as set forth in the table below. Each of these notes and accrued interest thereon may convert into shares of our common stock at the conversion price set forth in the table below. Certain of these notes were issued with detachable five-year warrants to purchase shares of our common stock as set forth in the table below.
| Issuance Date | Principal Amount | Original Issue Discount | Gross Proceeds | Interest Rate | Maturity Date | Note Conversion Price Per Share | Number of Shares Underlying Warrants | Warrant Exercise Price Per Share | ||||||||||||||||||||||||
| April 18, 2019 | $ | 150,000 | (1) | $ | - | $ | 150,000 | 10% | (1,2) | March 17, 2020 | (5) | $ | - | (1) | 2,500 | $ | 6.25 | (5) | ||||||||||||||
| July 19, 2019 | 815,217 | 65,217 | 750,000 | 8% | (3) | June 30, 2020 | (6) | 4.27 | (9,10) | 7,500 | 4.27 | (15) | ||||||||||||||||||||
| September 4, 2019 | 108,696 | 8,696 | 100,000 | 8% | (3) | June 30, 2020 | (6,7) | 24.00 | (11) | 1,000 | 24.00 | |||||||||||||||||||||
| September 25, 2019 | 54,348 | 4,348 | 50,000 | 8% | (3) | June 30, 2020 | (6,7) | 24.00 | (11) | 500 | 24.00 | |||||||||||||||||||||
| October 3, 2019 | 27,174 | 2,174 | 25,000 | 8% | (3) | June 30, 2020 | (6,7) | 24.00 | (11) | 250 | 24.00 | |||||||||||||||||||||
| October 10, 2019 | 27,174 | 2,174 | 25,000 | 8% | (3) | June 30, 2020 | (6,7) | 24.00 | (11) | 250 | 24.00 | |||||||||||||||||||||
| October 16, 2019 | 217,391 | 17,391 | 200,000 | 8% | (3) | June 30, 2020 | (6) | 4.27 | (9,10) | 2,000 | 4.27 | (15) | ||||||||||||||||||||
| October 23, 2019 | 108,696 | 8,696 | 100,000 | 8% | (3) | June 30, 2020 | (6,7) | 24.00 | (11) | 1,250 1,250 |
30.00 40.00 |
|||||||||||||||||||||
| October 29, 2019 | 27,174 | 2,174 | 25,000 | 8% | (3) | June 30, 2020 | (6,7) | 24.00 | (11) | 250 | 24.00 | |||||||||||||||||||||
| November 8, 2019 | 16,304 | 1,304 | 15,000 | 8% | (3) | June 30, 2020 | (6,7) | 14.00 | (11) | 150 | 14.00 | |||||||||||||||||||||
| November 15, 2019 | 100,000 | - | 100,000 | 14% | (3) | June 30, 2020 | (6,7) | 20.00 | (11) | - | (12) | - | ||||||||||||||||||||
| January 6, 2020 | 10,870 | 870 | 10,000 | 8% | (3) | June 30, 2020 | (6,7) | 10.00 | (11) | 100 | 10.00 | |||||||||||||||||||||
| January 21, 2020 | 262,500 | 12,500 | 250,000 | 10% | (2,4) | June 30, 2020 | (8) | 4.27 | (11) | - | (12,13) | - | ||||||||||||||||||||
| February 25, 2020 | 52,632 | 2,632 | 50,000 | 8% | (3) | June 30, 2020 | (6,7) | 7.50 | (10) | 500 | 7.50 | |||||||||||||||||||||
| March 16, 2020 | 250,000 | 20,000 | 230,000 | 10% | (2) | September 16, 2020 | (6) | 4.50 | (9,11) | - | (12,14) | - | ||||||||||||||||||||
| March 16, 2020 | 250,000 | 20,000 | 230,000 | 10% | (2) | September 16, 2020 | (6) | 4.50 | (9,11) | - | (12,14) | - | ||||||||||||||||||||
| Total | $ | 2,478,176 | $ | 168,176 | $ | 2,310,000 | 8-14% | 2020 | $ | 4.27-24.00 | 17,500 | $ | 4.27-40.00 | |||||||||||||||||||
| (1) | This note was fully repaid as of March 17, 2020. | |
| (2) | Accrued interest on this note is payable upon maturity. | |
| (3) | Accrued interest on this note is payable monthly in cash. | |
| (4) | One-time fixed interest charge equal to ten percent (10%). | |
| (5) | The maturity date of this note was extended from December 31, 2019 to March 31, 2020 pursuant to an agreement between the holder and us, under which we also agreed to make payments of accrued interest and principal, together with a prepayment penalty equal to twenty percent (20%) of the principal payment, in an aggregate amount of not less than $15,000 per month beginning February 1, 2020 until this note was repaid in full. On March 17, 2020, we fully repaid the remaining principal and interest due under this note, together with a reduced prepayment penalty equal to approximately ten percent (10%) of the principal payment, for an aggregate payment amount of $150,000, pursuant to an agreement between the holder and us, under which we also agreed to adjust the exercise price of the warrant issued in connection with this note to $6.25 per share. | |
| (6) | Prepayment of this note is not subject to a prepayment penalty or premium. | |
| (7) | If this note, or any portion thereof, has not been repaid or converted in full on or prior to the maturity date, then repayment of the unpaid principal balance plus any accrued and unpaid interest thereon, shall be amortized over the following thirty-six (36) months. | |
| (8) | Prepayment of this note is subject to a prepayment penalty or premium of fifteen percent (15%) for prepayments on or prior to March 31, 2020, twenty-five percent (25%) for prepayments on or prior to May 15, 2020, or thirty percent (30%) prior to June 30, 2020 | |
| (9) | The conversion price of this note is subject to adjustment upon the issuance of our common stock or securities convertible into our common stock at a price per share less than the then prevailing conversion price, other than specified exempt issuances. | |
| (10) | This note and accrued interest thereon may convert into shares of our common stock any time at the holder’s option or automatically upon a qualified financing of at least $5 million at the lower of the conversion price then in effect or a twenty-five percent (25%) discount to the offering price. | |
| (11) | This note and accrued interest thereon may convert into shares of our common stock any time at the holder’s option. | |
| (12) | No warrant was issued in connection with this note. | |
| (13) | 5,855 shares of our common stock were issued in connection with the purchase of this note. | |
| (14) | 5,000 shares of our common stock were issued as a commitment fee in connection with the purchase of this note. In addition, 27,777 shares of our common stock were issued in connection with the purchase of this note; provided, however, such shares must be returned to us if this note is fully repaid within six (6) months following the issuance date. | |
| (15) | The exercise price of this warrant shall be adjusted in accordance with any adjustment to the conversion price of this note. |
| 47 |
2019 Unit Issuances
On February 22, 2019, we sold to a current stockholder of the Company 334 units of our securities for an aggregate purchase price of $20,000. Each unit consisted of (i) two shares of our common stock, and (ii) a five-year warrant to purchase one share of our common stock at $40.00. No placement agent or broker dealer was used or participated in any offering or sale of such units.
On March 29, 2019, we sold to a current stockholder of the Company 417 units of our securities for an aggregate purchase price of $25,000. Each unit consisted of (i) two shares of our common stock, and (ii) a five-year warrant to purchase one share of our common stock at $40.00. No placement agent or broker dealer was used or participated in any offering or sale of such units.
On April 3, 2019, we sold to a current stockholder of the Company 1,667 units of our securities for an aggregate purchase price of $100,000. Each unit consisted of (i) two shares of our common stock, and (ii) a five-year warrant to purchase one share of our common stock at $40.00. No placement agent or broker dealer was used or participated in any offering or sale of such units.
On April 9, 2019, we sold to a current stockholder of the Company 1,667 units of our securities for an aggregate purchase price of $100,000. Each unit consisted of (i) two shares of our common stock, and (ii) a five-year warrant to purchase one share of our common stock at $40.00. No placement agent or broker dealer was used or participated in any offering or sale of such units.
Stock Option Exercise
On April 16, 2018, we issued 386 shares of our common stock in connection with the cashless exercise of stock options for 500 shares of our common stock at $12.00 per share with 114 shares of our common stock withheld with an aggregate fair market value equal to the aggregate exercise price.
On May 2, 2018, we issued 400 shares of our common stock in connection with the cashless exercise of stock options for 500 shares of our common stock at $12.00 per share with 100 shares of our common stock withheld with an aggregate fair market value equal to the aggregate exercise price.
Stock Based Compensation
During the year ended December 31, 2018, we issued 6,725 shares of our common stock to our independent directors for compensation and 563 shares of our common stock to service providers for compensation.
During the year ended December 31, 2019, we issued 11,054 shares of our common stock to our independent directors for compensation and 750 shares of our common stock to service providers for compensation.
On July 27, 2018, we issued warrants to purchase 1,579 shares of our common stock at an exercise price of $42.00 per share until July 27, 2023 to placement agents for compensation.
The following table sets forth options to purchase shares of our common stock we issued recently to employees, directors, and service providers for compensation.
| Issuance Date | Number of Shares Underlying Options | Option Exercise Price Per Share | Option Expiration Date | Option Vesting | ||||||||
| January 1, 2018 | 2,500 | $ | 32.00 | January 1, 2028 | (1) | |||||||
| January 1, 2018 | 1,668 | $ | 32.00 | January 1, 2023 | (2) | |||||||
| June 1, 2018 | 5,000 | $ | 48.00 | June 1, 2028 | (3) | |||||||
| November 14, 2018 | 5,000 | $ | 42.00 | November 14, 2028 | (4) | |||||||
| (1) | One-fourth of the shares vest one year from issuance and one forty-eighth of the shares vest monthly thereafter. | |
| (2) | The shares are fully vested. | |
| (3) | The shares vest monthly over two years. | |
| (4) | One-half of the shares vest monthly over four years and the remaining shares vest upon certain milestones. |
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
During each month within the fourth quarter of the fiscal year ended December 31, 2019, neither we nor any “affiliated purchaser,” as that term is defined in Rule 10b-18(a)(3) under the Exchange Act, repurchased any of our common stock or other securities.
Item 6. Selected Financial Data.
We are a “smaller reporting company,” and, accordingly, we are not required to provide the information required by this Item.
| 48 |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The financial data discussed below is derived from our audited consolidated financial statements for the fiscal years ended December 31, 2019 and 2018, which are found elsewhere in this Annual Report on Form 10-K. Our consolidated financial statements are prepared and presented in accordance with generally accepted accounting principles in the United States. The financial data discussed below is only a summary and investors should read the following discussion and analysis of our financial condition and results of our operations in conjunction with our consolidated financial statements and the related notes to those statements included elsewhere in this Annual Report on Form 10-K. This discussion contains forward-looking statements reflecting our current expectations that involve risks and uncertainties. Our actual results and the timing of events may differ materially from those contained in these forward-looking statements due to a number of factors, including those discussed in the section entitled “Risk Factors,” and elsewhere in this Annual Report on Form 10-K.
Corporate Overview and History
We are a development stage biopharmaceutical company primarily focused on the development of pharmaceuticals for chronic diseases driven by inflammation. We also have a commercial business unit that markets dietary supplements for inflammatory health. CDX-101, our astaxanthin pharmaceutical candidate, is being developed for cardiovascular inflammation and dyslipidemia, with a target initial indication of severe hypertriglyceridemia. CDX-301, our zeaxanthin pharmaceutical candidate, is being developed for macular degeneration, with a target initial indication of Stargardt disease. Our pharmaceutical candidates are currently in pre-clinical development, including the planning of IND enabling studies. ZanthoSyn® is a physician recommended astaxanthin dietary supplement for inflammatory health. We sell ZanthoSyn® primarily through wholesale and e-commerce channels. The safety and efficacy of our products have not been directly evaluated in clinical trials or confirmed by the FDA.
At present we are not able to estimate if or when we will be able to generate sustained revenues. Our financial statements have been prepared assuming that we will continue as a going concern; however, given our recurring losses from operations, our independent registered public accounting firm has determined there is substantial doubt about our ability to continue as a going concern.
Results of Operations
Results of Operations for the Years Ended December 31, 2019 and 2018
The following table reflects our operating results for the years ended December 31, 2019 and 2018:
| Operating Summary | Year ended December 31, 2019 | Year ended December 31, 2018 | Change | |||||||||
| Revenues, net | $ | 710,949 | $ | 1,510,875 | $ | (799,926 | ) | |||||
| Cost of Goods Sold | (345,393 | ) | (699,852 | ) | 354,459 | |||||||
| Gross Profit | 365,556 | 811,023 | (445,467 | ) | ||||||||
| Operating Expenses | (4,442,659 | ) | (4,833,518 | ) | 390,859 | |||||||
| Net Operating Loss | (4,077,103 | ) | (4,022,495 | ) | (54,608 | ) | ||||||
| Other Expenses, net | (1,015,934 | ) | (1,727 | ) | (1,014,207 | ) | ||||||
| Net Loss | $ | (5,093,037 | ) | $ | (4,024,222 | ) | $ | (1,068,815 | ) | |||
| 49 |
Operating Summary
Our revenues presently derive from the sale of ZanthoSyn® primarily through wholesale and, to a lesser extent, e-commerce channels. We launched our e-commerce channel in 2016 and began selling to GNC stores in 2017. ZanthoSyn® is currently available at over two thousand GNC corporate stores in the United States. As a result, revenues were $710,949 and $1,510,875 for the years ended December 31, 2019 and 2018, respectively. The decrease in revenues for the year ended December 31, 2019 was primarily attributed to decreased replenishment orders by GNC during the current period compared to the previous year. Costs of goods sold were $345,393 and $699,852 for the years ended December 31, 2019 and 2018, respectively, and included costs of the product, shipping and handling, sales taxes, merchant fees, and other costs incurred on the sale of goods. Gross profits were $365,556 and $811,023 for the years ended December 31, 2019 and 2018, which represented gross profit margins of 51% and 54%, respectively.
Operating expenses were $4,442,659 and $4,833,518 for the years ended December 31, 2019 and 2018, respectively. Operating expenses primarily consisted of services provided to the Company, including payroll, consultation, and contract services, for research and development, including our clinical trial and pharmaceutical development programs, sales and marketing, and administration. These expenses were paid in accordance with agreements entered into with each employee or service provider. Included in operating expenses were $708,588 and $650,271 in stock-based compensation for the years ended December 31, 2019 and 2018, respectively.
Other expenses, net, were $1,015,934 and $1,727 for the years ended December 31, 2019 and 2018, respectively. For the year ended December 31, 2019, other expenses, net, primarily consisted of interest expense of $623,415, change in the fair value of derivative liabilities of $356,314, and loss on the abandonment of patents of $36,205. For the year ended December 31, 2018, other expense primarily consisted of interest expense of $4,227, which was offset by interest and other income of $2,500.
Assets and Liabilities
Assets were $2,018,922 and $2,458,898 as of December 31, 2019 and 2018, respectively. The decrease was primarily due to decreases in cash and inventory. At December 31, 2019 and 2018, cash totaled $19,303 and $243,753, respectively. Negative working capital was $6,547,114 and $3,877,290 as of December 31, 2019 and 2018, respectively, and was primarily due to accrued payroll and paid time off of $3,687,376 and $3,428,011, accrued Board of Director fees and related consultation of $418,546, accounts payable of $1,544,402 and $1,996,097, and the aggregate current liability related to notes payable, convertible notes payable, and derivative liability on convertible notes payable, of $2,412,324 and $0, less current assets of $1,586,061 and $2,024,364, respectively. The issuance of convertible notes resulted in a derivative liability of $827,314 as of December 31, 2019.
The accrual of payroll and Board of Director fees and related consultation, which primarily occurred from January 2008 to December 2013 and during the quarter ended December 31, 2019, was due to significant capital constraints, and was selected in favor of layoffs or furloughs in order to maximize employee and director retention. In 2013 and 2014, the Company initiated repayment on these accrued amounts, utilizing approximately 5% to 10% of proceeds from various financings and plans to continue a structured repayment of the outstanding amounts over time as resources permit.
| 50 |
Liquidity and Capital Resources
Since our inception, we have sustained operating losses and have used cash raised by issuing securities. We expect to continue to operate with a net loss until we are able to develop and commercialize our pharmaceutical product candidates. During the years ended December 31, 2019 and 2018, we used cash in operating activities of $3,522,837 and $3,200,528, respectively, and incurred net losses of $5,093,037 and $4,024,222, respectively.
As of December 31, 2019, we had a U.S. federal income tax net operating loss (“NOL”) carryforward of approximately $41 million. These NOLs may be available to offset our future taxable income to the extent permitted under the Internal Revenue Code (the “IRC”). Under IRC Section 382, the use of NOL carryforwards, capital loss carryforwards, and other tax credit carryforwards may be significantly limited if a change in ownership of a company occurs. A change in ownership under IRC Section 382 is defined, generally, as a cumulative change of 50 percentage points or more in the ownership positions of certain stockholders owning 5% or more of a company’s common stock over a three-year rolling period. If we were to have a change of ownership within the meaning of IRC Section 382, then under certain conditions, our annual federal NOL utilization could be limited to an amount equal to our market capitalization (valued at the time of the ownership change) multiplied by the federal long-term tax exempt rate.
Our existing liquidity is not sufficient to fund our operations, including payroll, anticipated capital expenditures, working capital, and other financing requirements for the foreseeable future. We may require more financing than anticipated, especially if we experience downturns or cyclical fluctuations in our business that are more severe or longer than anticipated, or if we experience significant increases in the cost of manufacturing, research and development, or sales and marketing activities, or increases in our expense levels resulting from being a publicly-traded company.
Our working capital and capital requirements at any given time depend upon numerous factors, including, but not limited to:
| ● | revenues from the sale of any products or licenses; | |
| ● | costs of production, marketing and sales capabilities, or other operating expenses; and | |
| ● | costs of research, development, and commercialization of our products and technologies. |
We have undertaken certain actions regarding the advancement of our pharmaceutical development program, the conduct of a dietary supplement clinical trial, and the continued sales and marketing of our commercial dietary supplement. We plan to fund such activities, including compensation to service providers, with a combination of cash and equity payments. The amount of payments in cash and equity will be determined by us from time to time.
We will incur ongoing recurring expenses associated with professional fees for accounting, legal, and other expenses for annual reports, quarterly reports, proxy statements, and other filings under the Exchange Act. We estimate that these costs will likely be in excess of $250,000 per year. These obligations will reduce our ability and resources to fund other aspects of our business. We hope to be able to use our status as a public company to increase our ability to use non-cash means of settling obligations and compensate certain independent contractors who provide professional services to us, although there can be no assurances that we will be successful in any of those efforts.
| 51 |
We require additional financing in order to continue to fund our operations and to pay existing and future liabilities and other obligations.
During the years ended December 31, 2019 and 2018, we raised financing of $3,360,000 and $1,244,037, respectively. During the year ended December 31, 2019, our financing was through the issuance of $245,000 in common stock, $1,575,000 in notes payable to related parties, $1,050,000 in convertible notes payable to related parties, and $490,000 in convertible notes payable. In accordance with U.S. GAAP, derivative liabilities of $827,314 were recognized in connection with convertible notes outstanding as of December 31, 2019; however, these are non-cash amounts and do not directly impact our liquidity or capital needs.
We filed a registration statement on Form S-1 on August 14, 2019, as amended September 27, 2019 and November 22, 2019, for a proposed $15 million public offering of our common stock and warrants and the listing of our common stock and such warrants on the Nasdaq Capital Market (the “Proposed Public Offering”). We intend to use the proceeds from the Proposed Public Offering primarily to fund pharmaceutical development and our operations. After giving effect to the net proceeds that we would receive from the Proposed Public Offering, if closed, we expect to have sufficient cash resources to support our expected operations for at least one year. Notwithstanding the uncertain market conditions related to COVID-19, we plan to continue to take actions to consummate the Proposed Public Offering. We cannot give any assurance that the Proposed Public Offering will be consummated on acceptable terms, or at all. In addition, prior to any closing of the Proposed Public Offering, we will need to obtain additional financing, which may not be available on acceptable terms and conditions, or at all.
As of the date hereof, we have outstanding promissory notes (including notes payable, convertible notes payable, and secured convertible notes payable) that are (i) due June 30, 2020 in the aggregate principal amount of $2,403,176, (ii) due September 16, 2020 in the aggregate principal amount of $500,000, and (iii) due January 11, 2022 in the aggregate principal amount of $1,000,000. Certain promissory notes due June 30, 2020 in the aggregate principal amount of (i) $1,085,240 may convert into shares of our common stock any time at the holder’s option or automatically upon a qualified financing of at least $5 million, (ii) $742,936 may convert into shares of our common stock any time at the holder’s option, and (iii) $575,000 do not have a conversion feature. In addition, repayment of certain promissory notes due June 30, 2020 in the aggregate principal amount of $533,068 can be amortized over thirty-six (36) months if not repaid or converted in full on or prior to the maturity date; and repayment of a certain promissory note due June 30, 2020 in the aggregate principal amount of $262,500 is secured by our finished goods inventory and a personal guaranty of our Chief Executive Officer. Promissory notes due September 16, 2020 in the aggregate principal amount of $500,000 may convert into shares of our common stock any time at the holder’s option. Our ability to repay any and all of these notes as they become due if not otherwise repaid or converted on or prior to the maturity dates described above is uncertain and will be based on our ability to raise additional capital, generate additional revenues, and/or modify the terms of such debt instruments to the extent necessary.
We need additional capital to fund our operations and pay our current and future obligations, including without limitation our outstanding promissory notes; however, our ability to access the capital markets or otherwise raise such capital is unknown during the COVID-19 pandemic and there can be no assurance that we will be able to obtain sufficient amounts of capital as and when needed. Any additional financing in one or more transactions through the private placement of our common stock, warrants to purchase our common stock, debt, and/or convertible securities prior to any closing of the Proposed Public Offering or as an alternative thereto may not be available to us on acceptable terms and conditions, or at all.
Any inability to obtain additional financing will materially and adversely affect us, including requiring us to significantly curtail or cease business operations altogether. We cannot give any assurance that we will in the future be able to achieve a level of profitability from the sale of existing or future products or otherwise to sustain our operations. These conditions raise substantial doubt about our ability to continue as a going concern. The accompanying financial statements do not include any adjustments to reflect the possible future effects on recoverability and reclassification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
| 52 |
The following is a summary of our cash flows provided by (used in) operating, investing, and financing activities during the periods indicated:
| Cash Flow Summary | Year ended December 31, 2019 | Year ended December 31, 2018 | ||||||
| Net Cash from Operating Activities | $ | (3,522,837 | ) | $ | (3,200,528 | ) | ||
| Net Cash from Investing Activities | (61,613 | ) | (36,593 | ) | ||||
| Net Cash from Financing Activities | 3,360,000 | 1,244,037 | ||||||
| Net Cash (Decrease) Increase | (224,450 | ) | (1,993,084 | ) | ||||
| Cash at Beginning of Year | 243,753 | 2,236,837 | ||||||
| Cash at End of Year | $ | 19,303 | $ | 243,753 | ||||
Cash Flows from Operating Activities
During the years ended December 31, 2019 and 2018, our operating activities primarily consisted of receipts and receivables from sales, payments or accruals for employees, directors, and consultants for services related to research and development, sales and marketing, and administration, and deposits for future inventory.
Cash Flows from Investing Activities
During the years ended December 31, 2019 and 2018, our investing activities were primarily related to expenditures on patents.
Cash Flows from Financing Activities
During the years ended December 31, 2019 and 2018, our financing activities consisted of transactions in which we raised proceeds through the issuance of our common stock and convertible and other notes payable.
Recently Issued Accounting Pronouncements
In August 2018, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2018-13, Fair Value Measurement. This ASU modifies the disclosure requirements on fair value measurements in Topic 820, Fair Value Measurement, based on the concepts in the FASB’s Concepts Statement, including the consideration of costs and benefits. The guidance in ASU No. 2018-13 is effective for annual reporting periods, and interim periods within those years, beginning after December 15, 2019. The Company is currently in the process of evaluating the impact of the adoption of this ASU on its consolidated financial statements.
In November 2019, the FASB issued ASU No. 2019-08, Compensation—Stock Compensation (Topic 718) and Revenue from Contracts with Customers (Topic 606). The amendments in this Update require that an entity apply the guidance in Topic 718 to measure and classify share-based payment awards granted to a customer. The amount recorded as a reduction in the transaction price should be based on the grant-date fair value of the share-based payment award. The guidance in ASU No. 2019-08 is effective fiscal years beginning after December 15, 2019, and interim periods within fiscal years beginning after December 15, 2020. The Company is currently in the process of evaluating the impact of the adoption of this ASU on its consolidated financial statements.
In December 2019, the FASB Issued ASU No. 2019-12, Income Taxes (Topic 740) Simplifying the Accounting for Income Taxes. The amendments in this Update simplify the accounting for income taxes by removing certain exceptions to the general principles in Topic 740. The amendments also improve consistent application of and simplify U.S. GAAP for other areas of Topic 740 by clarifying and amending existing guidance. For public business entities, the amendments in this Update are effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020. The Company is currently in the process of evaluating the impact of the adoption of this ASU on its consolidated financial statements.
The Company does not believe that any other recently issued, but not yet effective accounting pronouncements, if adopted, would have a material effect on the consolidated financial statements.
Off-Balance Sheet Arrangements
There are no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures, or capital resources.
| 53 |
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
We are a “smaller reporting company,” and, accordingly, we are not required to provide the information required by this Item.
Item 8. Financial Statements and Supplementary Data.
The consolidated financial statements required by this Item, together with the report of our independent registered public accounting firm, KBL, LLP, begin on page F-1, immediately following the signatures to this annual report. Please refer to Item 15 of this report for an index of the consolidated financial statements included in this annual report.
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
Not applicable.
Item 9A. Controls and Procedures.
Disclosure Controls and Procedures
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 15d-15(f). Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that (a) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (b) provide reasonable assurance that transactions are recorded as necessary to permit the preparation of financial statements in accordance with generally accepted accounting principles and that receipts and expenditures of the Company are being made only in accordance with authorizations of the our management and directors; and (c) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements. Based on our evaluation under the framework in Internal Control—Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2019.
Changes in Internal Controls over Financial Reporting
There were no changes in the Company’s internal control over financial reporting during the fiscal year ended December 31, 2019, that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
Not applicable.
| 54 |
Item 10. Directors, Executive Officers, and Corporate Governance.
Set forth below is a list of the names, ages and positions of our directors and executive officers.
| Name | Age | Position(s) | ||
| George W. Bickerstaff, III | 64 | Chairman of the Board of Directors | ||
| David G. Watumull | 70 | President, Chief Executive Officer, and Director | ||
| Terence A. Kelly, Ph.D. | 58 | Director | ||
| Michele Galen | 63 | Director | ||
| Makarand Jawadekar, Ph.D. | 69 | Director | ||
| Elona Kogan | 50 | Director | ||
| David M. Watumull | 38 | Chief Operating Officer | ||
| John B. Russell | 47 | Chief Financial Officer and Treasurer |
Biographies of Directors and Executive Officers
George W. Bickerstaff, III has served as a Director since June 16, 2014. Mr. Bickerstaff is currently a partner and the managing director of M.M. Dillon & Co., a healthcare and technology investment bank that he co-founded. Previously, he served as Chief Financial Officer of Novartis Pharma AG from 2000 to 2005, held senior financial positions at IMS Health from 1989 to 1997 and held financial positions with Dun & Bradstreet and General Electric. Mr. Bickerstaff currently serves as a member of the boards of directors of the following public companies: Axovant Sciences Ltd., CareDx, Inc. and Innoviva, Inc. He also previously served on the board of directors of ARIAD Pharmaceuticals, Inc. and Inovio Pharmaceuticals, Inc. Mr. Bickerstaff received his B.S. in Engineering and his B.A. in Business Administration from Rutgers University. We believe that Mr. Bickerstaff’s experience in the pharmaceutical and biopharmaceutical industries and board leadership qualify him to serve on our Board of Directors.
David G. Watumull has served as our Chief Executive Officer, President, and Director since February 7, 2014. Mr. Watumull has served as the Chief Executive Officer, President, and Director of Cardax Pharma, Inc. since its inception in May 2013. Mr. Watumull also served as the Chief Executive Officer, President, and Director of Cardax Pharmaceuticals, Inc. from its inception in March 2006 until it merged with us in December 2015. Mr. Watumull is a co-founder of Cardax Pharmaceuticals, Inc. and has over 20 years of experience as a biotechnology industry executive. From 2001 to 2006, Mr. Watumull served as President, Chief Executive Officer, and Director of Hawaii Biotech, Inc. Mr. Watumull was Executive Vice President of Aquasearch, Inc., a public astaxanthin consumer health company, from 1998 to 2000. From 1997 to 1998 he headed his own biotech research firm, Watumull & Co. From 1994 to 1997 he was a biotech research analyst, money manager, and investment banker at First Honolulu Securities. From 1992 to 1994 he led his own money management firm, Biovest, Inc. Prior to that, from 1982 to 1992, Mr. Watumull worked at Paine Webber in various capacities, including as a biotech money manager and investment executive. Mr. Watumull studied mathematics at Claremont Men’s College (now Claremont McKenna College). Mr. Watumull’s extensive background in the biotechnology industry, his operational acumen, and his position of leadership since the founding of our business uniquely qualifies him to serve as a member of our Board of Directors.
Terence A. Kelly, Ph.D. has served as a Director since June 16, 2014. Dr. Kelly has over 25 years of experience as a scientist and executive in the pharmaceutical industry starting as a medicinal chemist in 1990. Dr. Kelly has been the President and Chief Executive Officer of Perception Neuroscience Holdings, Inc. since November 2019. Dr. Kelly also acts as a consultant to the biotech industry through his company, Kelly Pharma Research Consulting, LLC. From 2011 to 2017, Dr. Kelly was the President and Chief Executive Officer of CoMentis, Inc. From 1990 to 2009, Dr. Kelly served in various scientific and executive positions at Boehringer Ingelheim, where after a successful early scientific career, he was promoted to Vice President of its U.S.-based medicinal chemistry department, which included 145 scientists in the high throughput screening, computational chemistry, structural biology, combinatorial chemistry and medicinal chemistry groups. Dr. Kelly holds a B.S. degree in Chemistry from Rensselaer Polytechnic Institute (1982) and a Ph.D. degree in Chemistry from the University of Texas at Austin (1988). He completed postdoctoral work in natural products synthesis at Yale University (1988-1990) and holds an M.B.A. from New York University, Stern School of Business (1998). Dr. Kelly is the co-author of over 25 scientific publications and served on the College of Natural Sciences Advisory Council for the University of Texas. Dr. Kelly’s scientific training and his track record of delivering high quality compounds into advanced clinical studies provide valuable skills and knowledge to our Board of Directors.
| 55 |
Michele Galen has served as a Director since January 4, 2017. Ms. Galen serves as a strategic advisor and board member across pharmaceuticals, biotechnology, health start-ups and global health, drawing on her broad experience in global business, communications, law and journalism. From June 2016 to present, Ms. Galen has led an independent consultancy, Michele Galen LLC. From April 2015 to June 2016, Ms. Galen served as Global Head, Communications and Public Affairs, for Shire plc, a biotechnology company, where she served as the lead communications and public affairs advisor on the successful $32 billion acquisition and integration of Baxalta. From February 2015 to March 2015, Ms. Galen led an independent consultancy, Michele Galen LLC. From May 2014 to January 2015, Ms. Galen served as a senior advisor to Novartis AG. From February 2012 to May 2014, Ms. Galen led Global Communications for Novartis AG, based in Basel, Switzerland. From February 2010 to February 2012, Ms. Galen served as Vice President and Global Head of Communications & Patient Advocacy for Novartis Pharma AG. From October 2003 to February 2010, Ms. Galen served as Vice President and Global Head, Oncology Affairs for Novartis Pharma AG. From February 2001 to October 2003, Ms. Galen served as Vice President, Corporate Communications for Novartis Pharmaceuticals Corporation. Earlier in her career, Ms. Galen was a Managing Director in the global public relations firm Burson-Marsteller. There, she co-founded the Organizational Change Communications practice. She is an award-winning journalist, and worked as Legal Editor and Social Issues Editor at Business Week magazine. Ms. Galen is a member of the New York State Bar and practiced law at Stroock, Stroock & Lavan LLP, and Skadden, Arps, Slate, Meagher & Flom LLP. Ms. Galen currently serves on the boards of Symphony Space and IYNAUS US. She formerly served on the advisory board of MK&A, Global Oncology, Stupid Cancer, and the Global Health Council. Ms. Galen received a B.A. from George Washington University, M.S. from the Columbia University Graduate School of Journalism, and J.D. from New York University School of Law. She also received a certification in executive coaching from Columbia University. Ms. Galen’s broad pharmaceutical, biotechnology, and healthcare background provide valuable skills and knowledge to our Board of Directors.
Makarand Jawadekar, Ph.D. has served as a Director since June 1, 2018. Dr. Jawadekar is a pharmaceutical executive with over 35 years of experience focused on research and development. From October 2017 to present, Dr. Jawadekar has served as Director and Chief Science Officer of Preveceutical Medical Inc., a Canadian pharmaceutical research and development company. Dr. Jawadekar also serves as a strategic advisor to pharmaceutical and biotechnology companies through his independent consultancy, Melinda Consulting, LLC, which he founded in 2010. From 1982 to 2010, Dr. Jawadekar held various technical, management, and business development positions at Pfizer, Inc., including Director, Portfolio Management & Analytics, and Vice President, Asia Colleague Resource Group, for Pfizer Global R&D. Dr. Jawadekar received his B.Pharm. from Shivaji University (1972), M.Pharm. from the University of Bombay (1974), and Ph.D. in Pharmaceutics from the University of Minnesota (1982). Dr. Jawadekar’s academic and professional background in pharmaceuticals provides valuable knowledge and experience to our Board of Directors.
Elona Kogan has served as a Director since June 1, 2018. Ms. Kogan is a biotechnology executive with over 20 years of experience focused on building fast growing publicly traded companies in regulated industries. Ms. Kogan currently serves as the General Counsel & Corporate Secretary of Selecta Biosciences, a clinical stage biopharmaceutical company. Previously, Ms. Kogan served as the General Counsel & Senior Vice President of Government Relations for ARIAD Pharmaceuticals, Inc., a Cambridge, Massachusetts based biotechnology company, from July 2016 through May 2017. Prior to joining ARIAD, Ms. Kogan served as the Vice President of Legal Affairs, and subsequently head of Government Relations, for Avanir Pharmaceuticals, Inc., a California based biotechnology company, during the period of May 2011 through September 2015. Prior roles included positions at King Pharmaceuticals, Inc., Bristol-Meyers Squibb, and Bergen Brunswig Corporation. Ms. Kogan is a graduate of the Southwestern Law School SCALE Program. Ms. Kogan graduated cum laude from Columbia University, Barnard College, with a B.A. degree in Economics. Ms. Kogan’s professional experience working with publicly traded companies in the biotechnology and healthcare arena provides valuable skills and experience to our Board of Directors.
| 56 |
David M. Watumull has served as our Chief Operating Officer since August 2017 and previously as our Vice President, Operations from February 7, 2014 to August 2017. Mr. Watumull has also served as our Secretary since March 30, 2020 and previously as our Assistant Secretary since February 7, 2014. Mr. Watumull has also served as our Assistant Treasurer since February 7, 2014. Mr. Watumull has served as the Chief Operating Officer of Cardax Pharma, Inc. since December 2017 and previously as Vice President, Operations of Cardax Pharma, Inc. from its inception in May 2013 to December 2017. Mr. Watumull has also served as Assistant Treasurer and Assistant Secretary of Cardax Pharma, Inc. since July 2013 and previously as Secretary and Treasurer of Cardax Pharma, Inc. from May 2013 to July 2013. Mr. Watumull also served as Vice President, Operations, Assistant Treasurer, and Assistant Secretary of Cardax Pharmaceuticals, Inc. from July 2013 until it merged with us in December 2015, and previously as Director, Operations and Finance from 2009 to 2013, Operations Manager from 2008 to 2009, and Program Manager from its inception in 2006 to 2009. Mr. Watumull oversees all operations with responsibility for product development and manufacturing, regulatory compliance, sales and marketing, finance, and administration. Mr. Watumull was previously Program Manager at Hawaii Biotech, Inc. from 2005 to 2006, Project Coordinator from 2004 to 2005, and Information Technology Associate / Manager from 2002 to 2004. Mr. Watumull also worked at Aquasearch, Inc., from 2000 to 2001 in various capacities including Medical Information Specialist and Information Technology Associate. Mr. Watumull studied Electrical Engineering at the University of Hawaii.
John B. Russell, CPA has served as our Chief Financial Officer and Treasurer since February 7, 2014. Mr. Russell has served as the Chief Financial Officer and Treasurer of Cardax Pharma, Inc. since July 2013. Mr. Russell also served as the Chief Financial Officer and Treasurer of Cardax Pharmaceuticals, Inc. from July 2013 until it merged with us in December 2015. Mr. Russell is the founder of JBR Business Solutions, LLC and has served as its President since 2010. Mr. Russell has over 20 years of accounting, finance, operations, and SEC reporting experience in biopharmaceutical and high-tech industries. From 2010 to the present, he has served as Chief Financial Officer for various privately-held start-up companies. Mr. Russell was in charge of the Business Advisory Services for the Grant Thornton Honolulu office from 2006 to 2010. From 2005 to 2006, Mr. Russell worked at a consulting company as the Operations Consulting - Financial Management lead, advising Cisco Systems, Inc. Mr. Russell was the General Accounting Manager of the publicly traded company Scios Inc. from 2003 to 2005, where he was in charge of SEC reporting and internal controls. Mr. Russell was the Controller for several portfolio companies in the venture capital firm, Raza Foundries, Inc., from 2001 to 2002, and the General Accounting Manager for inSilicon Corporation, a public company, from 2000 to 2001. Previous to that, Mr. Russell was an auditor at PricewaterhouseCoopers LLP from 1995 to 2000. Mr. Russell is a licensed CPA in Hawaii and has a B.A. in Economics/Accounting from Claremont McKenna College.
Executive officers are appointed by our Board of Directors. Each executive officer holds his or her office until he or she resigns, is removed by our Board of Directors or his or her successor is elected and qualified. Directors are elected annually by our stockholders at the annual meeting. Each director holds his or her office until his or her successor is elected and qualified or his or her earlier resignation or removal.
There have been no material changes to the procedures by which security holders may recommend nominees to our Board of Directors since our last annual report.
| 57 |
Scientific Advisory Board and Key Scientific Personnel
We have assembled a Scientific Advisory Board (“SAB”) and key scientific personnel with expertise in science and medicine with significant experience in pharmaceutical development applicable to our strategy. The members of our SAB and key scientific personnel have made significant scientific contributions in their individual fields, have published in top-tier journals, and have been recognized with numerous awards and distinctions. Our SAB meets on an as-needed basis, based on our need for advice in their respective fields of expertise from time to time. The members of our Scientific Advisory Board and our key scientific personnel are:
| Name | Affiliation, Position | |
| Deepak L. Bhatt, M.D., M.P.H. | Cardax, Chairman of Scientific Advisory Board Harvard Medical School affiliated Brigham and Women’s Hospital, Executive Director of Interventional Cardiovascular Programs Harvard Medical School, Professor | |
| Paresh N. Soni, M.D., Ph.D. | Cardax, Chief Clinical and Regulatory Strategist Cardax, Member of Scientific Advisory Board | |
| R. Preston Mason, Ph.D. | Cardax, Member of Scientific Advisory Board Harvard Medical School-affiliated Brigham and Women’s Hospital, Department of Medicine, Division of Cardiology, Faculty | |
| Gilbert M. Rishton, Ph.D. | Cardax, Chief Science Officer | |
| Jon L. Ruckle, M.D. | Cardax, Chief Medical Officer | |
| Timothy J. King, Ph.D. | Cardax, Vice President, Research |
Deepak L. Bhatt, M.D., M.P.H. has served as Chairman of our Scientific Advisory Board since 2007. Dr. Bhatt has been the Executive Director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital Heart & Vascular Center since December 2013 and Professor of Medicine at Harvard Medical School since June 2012. Dr. Bhatt has authored or co-authored over 1,250 publications and is listed as a Thomson Reuters/Clarivate Analytics Highly Cited Researcher. He is the Editor of Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease and Atherothrombosis in Clinical Practice published by Oxford University Press. He is Senior Associate Editor for News and Clinical Trials for ACC.org. He is the Editor of the peer-reviewed Journal of Invasive Cardiology and Editor-in-Chief of the Harvard Heart Letter for patients. Previously, he was the Chief of Cardiology at VA Boston Healthcare System from 2008 to 2013. He also served as Associate Director of the Cardiovascular Coordinating Center from 2006 to 2008, Associate Director of the Cardiovascular Medicine Fellowship from 2001 to 2005, and Director of the Interventional Cardiology Fellowship from 2002 to 2005 at Cleveland Clinic, where he also served as an interventional cardiologist and an Associate Professor of Medicine. He also completed fellowships in interventional cardiology and cerebral and peripheral vascular intervention and served as Chief Interventional Fellow at Cleveland Clinic. Dr. Bhatt has been listed in Best Doctors in America from 2005 to 2018. He received the Eugene Braunwald Teaching Award for Excellence in the Teaching of Clinical Cardiology from Brigham and Women’s Hospital in 2017 and the ACC’s Distinguished Mentor Award in 2018. Dr. Bhatt’s research interests include acute coronary syndromes, preventive cardiology, and advanced techniques in cardiac, cerebral, and peripheral intervention. Dr. Bhatt obtained his undergraduate science degree as a National Merit Scholar at the Massachusetts Institute of Technology while also serving as a research associate at Harvard Medical School. He received his medical doctorate from Cornell University. His internship and residency in internal medicine were performed at the Hospital of the University of Pennsylvania. Dr. Bhatt also received a Master in Public Health with a concentration in clinical effectiveness from Harvard University.
| 58 |
Paresh N. Soni, M.D., Ph.D. has served as our Chief Clinical and Regulatory Strategist and as a member of Scientific Advisory Board since November 2018. Dr. Soni brings over 20 years of experience working with large and emerging pharmaceutical companies. He has led multidisciplinary teams across the entire drug development spectrum, from translational medicine to successful approval. Dr. Soni serves as a strategic advisor to pharmaceutical and biotechnology companies through his independent consultancy, Soni Biopharma Consulting, LLC, which he founded in September 2018. Previously, Dr. Soni served as Senior Vice President and Head of Development at Amarin Corporation from September 2008 to August 2013, where he led the development and regulatory approval of Vascepa for severe hypertriglyceridemia, and the design and launch of the landmark REDUCE-IT trial for cardiovascular prevention. Prior to joining Amarin, Dr. Soni worked at Pfizer, Inc. from 1999 to 2008, where he held a number of leadership roles in both experimental medicine and late stage development, including the submission of two New Drug Applications. Dr. Soni also served as the Chief Medical Officer of Albireo, a clinical-stage biopharmaceutical company, from September 2016 to August 2018, and as Vice President, Global Medical Sciences and Research at Alexion Pharmaceuticals, from June 2014 to July 2016. Dr. Soni is a member of the American Association for the Study of Liver Diseases. He has authored or co-authored more than 50 scientific papers in peer-reviewed journals, in addition to numerous abstracts. Dr. Soni is a board-certified internist and gastroenterologist. He completed his medical and specialist training at the University of Natal in South Africa. He also completed a research fellowship at the Division of Hepatology, Royal Free Hospital School of Medicine, London, where he received his Ph.D.
R. Preston Mason, Ph.D. has served as a member of Scientific Advisory Board since 2007. Dr. Mason has been on the faculty of the Department of Medicine, Division of Cardiology, at the Harvard Medical School affiliated Brigham and Women’s Hospital since 2002. He is also the President of Elucida Research LLC, a private biotechnology firm he founded in 2001. Previously, he was an associate professor at Drexel University College of Medicine from 1994 to 2001. He served as an assistant professor at the University of Connecticut Health Center from 1989 to 1993. Dr. Mason has published over 250 scientific research articles, book chapters, and abstracts and serves as a reviewer for numerous journals and scientific organizations, including the NIH. He has been the recipient of many awards and patents for his research in cardiovascular pharmacology, including an honorary doctorate in science. Dr. Mason is also a frequent lecturer at national and international meetings. Dr. Mason received his Bachelor of Science degree Summa Cum Laude from Gordon College in 1985. He received his PhD in cell biology and biophysics from the University of Connecticut Health Center in 1989.
| 59 |
Gilbert M. Rishton, Ph.D. has served as our Chief Science Officer since 2009. Dr. Rishton has been the Co-Founder and Chief Chemist at Cognition Therapeutics since January 2007. He was the Founder and Director of the Channel Islands Alzheimer’s Institute, a nonprofit whose mission is to enable new drug development through the identification of high-quality novel drug leads that might become Alzheimer’s disease medicines of the future, from 2004 to 2010. From 1995-2004, he served as a medicinal chemist at Amgen’s Thousand Oaks site, where he was responsible for initiating and building the Amgen Small Molecule Drug Discovery Group, which has grown to become one of the most formidable in the pharmaceutical industry. He also served as the chemistry manager for Amgen’s Sensipar development program, which spanned several phases from preclinical development to manufacturing and then human clinical trials, resulting in the commercial launch of Sensipar, Amgen’s first orally administered small molecule product. He also led the medicinal chemistry program for Amgen’s Secretase Team, which was among the first to produce small molecule secretase inhibitors as potential therapeutic agents for Alzheimer’s disease. Dr. Rishton obtained his undergraduate chemistry degree at University of Rhode Island (1983). He received his Ph.D. degree with a concentration in organic chemistry, organic synthesis, and morphinan synthesis from Florida State University (1988). Dr. Rishton was also a post-doctoral researcher at UC Irvine from 1989-1990.
Jon L. Ruckle, M.D. has acted as our Chief Medical Officer and in related medical advisory roles for us since 2013. Dr. Ruckle is a physician with over 20 years of full-time experience in clinical pharmacology research as an Investigator (over 350 studies), Medical Director of clinical research units devoted to Phase I studies, and consultant. Consulting activities focus on clinical study design for first-in-human through proof of concept studies, protocol development and writing, and medical monitoring. As Principal of Pacific Pharma Group, LLC, which Dr. Ruckle founded in 2008, he provides consultation services for the pharmaceutical and nutraceutical industry, including study design, product development strategy, and medical monitoring. Dr. Ruckle served as the founding Medical Director at Northwest Kinetics in Tacoma, WA, 1995 to 2000 (later acquired by Charles River Labs and subsequently by Comprehensive Clinical Development), then led Phase I development at Radiant Research Honolulu from 2000 until acquired by Covance in 2006, remained as Medical Director at Covance Honolulu to 2008, then founded Pacific Pharma Group to provide consulting services, and also served as Medical Director, Early Development for Comprehensive Clinical Development in Tacoma WA from 2011 to 2013.
Timothy J. King, Ph.D. has served as our Vice President, Research since 2009 and previously as our Senior Director of Biological Research from 2007 to 2009 and Director of Biological Research from 2006 to 2007. Dr. King is an expert on the mechanism of action and biological applications of astaxanthin and related carotenoids. Dr. King was the Director of Cancer Chemoprevention at Hawaii Biotech, Inc. from 2005-2006. From 2003-2005, he served as a Staff Scientist at the Fred Hutchinson Cancer Research Center, where he also served as a post-doctoral researcher from 1999 to 2004. Dr. King has over 25 years of combined academic and private sector scientific research experience including utilizing cell culture and animal model systems to address a wide range of topics including cardiovascular disease, liver disease, thrombosis, and cancer. Dr. King received his undergraduate degree in Biochemistry/Cell Biology from University of California San Diego. He obtained his Master of Science in biology (Molecular Virology) from San Diego State University studying Rhadoviral transcription processes in 1993. In 1999, he obtained his Ph.D. in Genetics/Molecular Biology from the University of Hawaii at Manoa where he focused on the tumor suppressor and growth regulatory roles of gap junction proteins using cancer cell culture systems and mouse tumor models. Concurrently, he also studied the influence of retinoids and various carotenoids on normal and tumor cell growth/behavior, carcinogenesis and gene regulation.
Family Relationships
David G. Watumull is the father of David M. Watumull. There are no other family relationships among any of our officers or directors.
| 60 |
Involvement in Certain Legal Proceedings
To the best of our knowledge, none of our directors or executive officers have been convicted in a criminal proceeding, excluding traffic violations or similar misdemeanors, or has been a party to any judicial or administrative proceeding during the past ten years that resulted in a judgment, decree, or final order enjoining the person from future violations of, or prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws, except for matters that were dismissed without sanction or settlement. Except as set forth in our discussion below in “Certain Relationships and Related Transactions, and Director Independence – Transactions with Related Persons,” none of our directors, director nominees, or executive officers has been involved in any transactions with us or any of our directors, executive officers, affiliates, or associates which are required to be disclosed pursuant to the rules and regulations of the Commission.
Code of Ethics
Our Code of Business Conduct and Ethics, effective as of February 7, 2014 (the “Code of Ethics”), contains the ethical principles by which our Chief Executive Officer and Chief Financial Officer, among others, are expected to conduct themselves when carrying out their duties and responsibilities. A copy of our Code of Ethics may be found on our website at www.cardaxpharma.com. We will provide a copy of our Code of Ethics to any person, without charge, upon request, by writing to David G. Watumull, Cardax, Inc., 2800 Woodlawn Drive, Suite 129, Honolulu, Hawaii 96822.
Board Committees
We are not required under the Securities and Exchange Act to maintain any committees of our Board of Directors. We have formed certain committees of our board as a matter of preferred corporate practices.
We have an audit committee, a compensation committee, and a nominating and corporate governance committee, each of which has the composition and responsibilities described below.
Audit Committee. Our audit committee oversees a broad range of issues surrounding our accounting and financial reporting processes and audits of our consolidated financial statements, including the following: (i) monitors the integrity of our financial statements, our compliance with legal and regulatory requirements, our independent registered public accounting firm’s qualifications and independence, and the performance of our internal audit function and independent registered public accounting firm; (ii) assumes direct responsibility for the appointment, compensation, retention and oversight of the work of any independent registered public accounting firm engaged for the purpose of performing any audit, review or attest services and for dealing directly with any such accounting firm; (iii) provides a medium for consideration of matters relating to any audit issues; and (iv) prepares the audit committee report that the rules require be included in our filings with the SEC. The members of our audit committee are Terence A. Kelly, Ph.D. (Chairperson), Makarand Jawadekar, Ph.D., and Elona Kogan. Our audit committee has a written charter available on our website at www.cardaxpharma.com.
Compensation Committee. Our compensation committee reviews and recommends policy relating to compensation and benefits of our officers, directors and employees, including reviewing and approving corporate goals and objectives relevant to the compensation of our Chief Executive Officer and other senior officers, evaluating the performance of these persons in light of those goals and objectives and setting compensation of these persons based on such evaluations. The compensation committee reviews and evaluates, at least annually, the performance of the compensation committee and its members, including compliance of the compensation committee with its charter. The members of our compensation committee are Elona Kogan (Chairperson), Makarand Jawadekar, Ph.D., and Michele Galen. Our compensation committee has a written charter available on our website at www.cardaxpharma.com.
Nominating and Corporate Governance Committee. The nominating and corporate governance committee oversees and assists our Board of Directors in identifying, reviewing and recommending nominees for election as directors; evaluating our Board of Directors and our management; developing, reviewing and recommending corporate governance guidelines and a corporate code of business conduct and ethics; and generally advises our Board of Directors on corporate governance and related matters. The members of our nominating and corporate governance committee are Michele Galen (Chairperson), Makarand Jawadekar, Ph.D., and Terence A. Kelly, Ph.D. Our nominating and corporate governance committee has a written charter available on our website at www.cardaxpharma.com.
| 61 |
Conflicts of Interest
Certain potential conflicts of interest are inherent in the relationships between our officers and directors and us.
From time to time, one or more of our affiliates may form or hold an ownership interest in and/or manage other businesses both related and unrelated to the type of business that we own and operate. These persons expect to continue to form, hold an ownership interest in and/or manage additional other businesses which may compete with our business with respect to operations, including financing and marketing, management time and services and potential customers. These activities may give rise to conflicts between or among the interests of us and other businesses with which our affiliates are associated. Our affiliates are in no way prohibited from undertaking such activities, and neither us nor our stockholders will have any right to require participation in such other activities.
Further, because we intend to transact business with some of our officers, directors and affiliates, as well as with firms in which some of our officers, directors or affiliates have a material interest, potential conflicts may arise between the respective interests of us and these related persons or entities. We believe that such transactions will be effected on terms at least as favorable to us as those available from unrelated third parties.
With respect to transactions involving real or apparent conflicts of interest, we have adopted policies and procedures which require that: (i) the fact of the relationship or interest giving rise to the potential conflict be disclosed or known to the directors who authorize or approve the transaction prior to such authorization or approval; and (ii) the transaction be fair and reasonable to us at the time it is authorized or approved by our directors.
| 62 |
Item 11. Executive Compensation.
Compensation of Executive Officers
The following sets forth information with respect to the compensation awarded or paid to David G. Watumull, our Chief Executive Officer, and David M. Watumull, our Chief Operating Officer, for all services rendered in all capacities to the Company and its predecessors during the fiscal years ending December 31, 2019 and 2018. These executive officers are referred to as the “named executive officers” throughout this report. In addition, the following sets forth information with respect to the compensation awarded or paid to our two highest compensated individuals not serving as executive officers, Gilbert M. Rishton, our Chief Science Officer, and Timothy J. King, our Vice President of Research, for all services rendered in all capacities to the Company and its predecessors during the fiscal years ending December 31, 2019 and 2018.
The following table sets forth information regarding each element of compensation provided to our named executive officers, and our two highest compensated individuals not serving as executive officers, for the fiscal years ended December 31, 2019 and 2018:
| Name | Year | Salary Paid(1) |
Salary Accrued(2) |
Salary Total |
Other Comp. (3) | Total | ||||||||||||||||
| David G. Watumull | 2019 | $ | 158,654 | $ | 28,846 | $ | 187,500 | $ | 11,151 | $ | 198,651 | |||||||||||
| Chief Executive Officer | 2018 | $ | 187,500 | $ | - | $ | 187,500 | $ | 10,534 | $ | 198,034 | |||||||||||
| David M. Watumull | 2019 | $ | 126,923 | $ | 23,077 | $ | 150,000 | $ | 6,217 | $ | 156,217 | |||||||||||
| Chief Operating Officer | 2018 | $ | 150,000 | $ | - | $ | 150,000 | $ | 7,443 | $ | 157,443 | |||||||||||
| Gilbert M. Rishton | 2019 | $ | 107,885 | $ | 19,615 | $ | 127,500 | $ | 936 | $ | 128,436 | |||||||||||
| Chief Science Officer | 2018 | $ | 127,500 | $ | - | $ | 127,500 | $ | 1,058 | $ | 128,558 | |||||||||||
| Timothy J. King | 2019 | $ | 107,885 | $ | 19,615 | $ | 127,500 | $ | 13,078 | $ | 140,578 | |||||||||||
| Vice President, Research | 2018 | $ | 127,500 | $ | - | $ | 127,500 | $ | 11,500 | $ | 139,000 | |||||||||||
| (1) | The amounts disclosed refer to salary paid in cash. | |
| (2) | The amounts disclosed refer to salary accrued during the year and outstanding as of year-end. | |
| (3) | The amounts disclosed refer to (i) imputed income in connection with certain benefits and/or insurance premiums paid in lieu of additional cash compensation, or (ii) other compensation paid in cash or accrued during the year and outstanding as of year-end. |
| 63 |
Outstanding Equity Awards to Executive Officers at Fiscal Year-End 2019
The following table sets forth information regarding outstanding option awards to our named executive officers as of December 31, 2019:
| Option awards(1)(2) | ||||||||||||||||||
| Name | Number of securities underlying unexercised options exercisable | Number of securities underlying unexercised options unexercisable | Number of securities underlying unexercised unearned options | Option exercise price ($) | Option expiration date | |||||||||||||
| David G. Watumull | 8,754 | - | - | $ | 31.00 | February 7, 2024 | ||||||||||||
| David G. Watumull | 24,710 | - | - | $ | 125.00 | February 7, 2024 | ||||||||||||
| David G. Watumull | 2,343 | (3) | - | - | $ | 64.00 | June 30, 2020 | |||||||||||
| David G. Watumull | 1,954 | (3) | - | - | $ | 40.00 | June 30, 2020 | |||||||||||
| David G. Watumull | 448 | (3) | - | - | $ | 98.00 | September 30, 2020 | |||||||||||
| David G. Watumull | 689 | (3) | - | - | $ | 54.00 | December 31, 2020 | |||||||||||
| David G. Watumull | 3,872 | (3) | - | - | $ | 12.00 | March 31, 2021 | |||||||||||
| David M. Watumull | 226 | - | - | $ | 31.00 | February 7, 2024 | ||||||||||||
| David M. Watumull | 11,943 | - | - | $ | 125.00 | February 7, 2024 | ||||||||||||
| David M. Watumull | 805 | (3) | - | - | $ | 64.00 | June 30, 2020 | |||||||||||
| David M. Watumull | 1,425 | (3) | - | - | $ | 40.00 | June 30, 2020 | |||||||||||
| David M. Watumull | 339 | (3) | - | - | $ | 98.00 | September 30, 2020 | |||||||||||
| David M. Watumull | 521 | (3) | - | - | $ | 54.00 | December 31, 2020 | |||||||||||
| David M. Watumull | 2,815 | (3) | - | - | $ | 12.00 | March 31, 2021 | |||||||||||
| (1) | The type of securities underlying all outstanding option awards is our common stock. | |
| (2) | None of our named executive officers have received stock awards. | |
| (3) | Stock options awarded in lieu of cash compensation. |
| 64 |
Compensation of Directors
The following table sets forth information regarding each element of compensation that we paid or awarded to our independent directors for the fiscal years ended December 31, 2019 and 2018:
| Name | Year | Cash Comp. | Equity Awards | Total(1) | ||||||||||
| George W. Bickerstaff, III | 2019 | $ | - | $ | 75,000 | (2) | $ | 75,000 | ||||||
| George W. Bickerstaff, III | 2018 | $ | - | $ | 75,000 | $ | 75,000 | |||||||
| Terence A. Kelly, Ph.D. | 2019 | $ | 25,000 | (3) | $ | 50,000 | (4) | $ | 75,000 | |||||
| Terence A. Kelly, Ph.D. | 2018 | $ | 25,000 | $ | 50,000 | $ | 75,000 | |||||||
| Michele Galen | 2019 | $ | - | $ | 75,000 | (2) | $ | 75,000 | ||||||
| Michele Galen | 2018 | $ | - | $ | 75,000 | $ | 75,000 | |||||||
| Makarand Jawadekar, Ph.D. | 2019 | - | 75,000 | (2) | 75,000 | |||||||||
| Makarand Jawadekar, Ph.D. | 2018 | $ | - | $ | 43,750 | (5) | $ | 43,750 | ||||||
| Elona Kogan | 2019 | - | 75,000 | (2) | 75,000 | |||||||||
| Elona Kogan | 2018 | $ | - | $ | 43,750 | (6) | $ | 43,750 | ||||||
| (1) | The amounts disclosed represent compensation in connection with services provided by each independent director. Each independent director receives quarterly compensation of $18,750 in arrears. Independent director compensation is payable in the form of a grant of shares of our common stock or non-qualified stock options to purchase shares of our common stock based on the higher of the then current market price or $30.00 per share, with up to one-third payable in cash at the election of the director. |
| (2) | Includes $18,750 for services provided during the quarter ended December 31, 2019, for which the shares were delivered in January 2020. |
| (3) | Includes $6,250 per quarter ($12,500 in aggregate) for services provided during the quarters ended September 30, 2019 and December 31, 2019, for which the cash compensation was accrued and outstanding as of year-end. |
| (4) | Includes $12,500 for services provided during the quarter ended December 31, 2019, for which the shares were delivered in January 2020. |
| (5) | Dr. Jawadekar was elected to the Board of Directors on June 1, 2018. Compensation was prorated accordingly. |
| (6) | Ms. Kogan was elected to the Board of Directors on June 1, 2018. Compensation was prorated accordingly. |
| 65 |
Outstanding Equity Awards to Directors at Fiscal Year-End 2019
The following table sets forth information regarding outstanding equity awards to our independent directors as of December 31, 2019:
| Stock awards(1) | Option awards (2) | |||||||||||||||||||||||
| Name | Number
of securities awarded | Number
of securities underlying unexercised options exercisable | Number
of securities underlying unexercised options unexercisable | Number
of securities underlying unexercised unearned options | Option exercise price ($) | Option
expiration date | ||||||||||||||||||
| George W. Bickerstaff, III | 10,153 | (3) | - | - | - | $ | - | - | ||||||||||||||||
| Terence A. Kelly, Ph.D. | 5,562 | (4) | - | - | - | $ | - | - | ||||||||||||||||
| Terence A. Kelly, Ph.D. | - | 2,084 | - | - | $ | 12.00 | March 31, 2021 | |||||||||||||||||
| Terence A. Kelly, Ph.D. | - | 139 | - | - | $ | 30.00 | September 30, 2021 | |||||||||||||||||
| Terence A. Kelly, Ph.D. | - | 417 | - | - | $ | 30.00 | December 31, 2021 | |||||||||||||||||
| Terence A. Kelly, Ph.D. | - | 391 | - | - | $ | 37.00 | March 31, 2022 | |||||||||||||||||
| Terence A. Kelly, Ph.D. | - | 417 | - | - | $ | 40.00 | June 30, 2022 | |||||||||||||||||
| Michele Galen | 5,673 | (3) | - | - | - | $ | - | - | ||||||||||||||||
| Makarand Jawadekar, Ph.D. | 3,448 | (3) | - | - | - | $ | - | - | ||||||||||||||||
| Elona Kogan | 3,448 | (3) | - | - | - | $ | - | - | ||||||||||||||||
| (1) | All shares are fully vested. | |
| (2) | The type of securities underlying all outstanding option awards is our common stock. | |
| (3) | Includes 625 shares for services provided during the quarter ended December 31, 2019, for which the shares were delivered in January 2020. | |
| (4) | Includes 417 shares for services provided during the quarter ended December 31, 2019, for which the shares were delivered in January 2020. |
| 66 |
Employment and Consulting Agreements
Executive Officer Compensation
On February 7, 2014, we entered into employment agreements with each of Messrs. David G. Watumull, David M. Watumull, Gilbert M. Rishton, and Timothy J. King, which provided for employment for an initial term of one year, subject to renewal and earlier termination rights as provided in such agreements. These agreements provide for compensation terms and duration of employment as set forth in each such agreement. Such agreements include restrictive covenants concerning competition with us and solicitation of our employees and clients, if such individuals are terminated for cause as defined in such agreements.
| ● | To conserve cash resources while seeking additional financing, we and our employees, including Messrs. David G. Watumull, David M. Watumull, Gilbert M. Rishton, and Timothy J. King, agreed to reduce cash compensation effective January 15, 2015. | |
| ● | On June 30, 2015, the compensation arrangements of Messrs. David G. Watumull, David M. Watumull, Gilbert M. Rishton, and Timothy J. King were amended so that, effective after June 30, 2015, we had the right to pay any compensation due to such officer during any calendar quarter that was not paid in cash in the form of shares of our common stock or incentive stock options under the 2014 Plan. In addition, the amount of the unpaid cash compensation that accrued during the first and second quarters of 2015 was paid with incentive stock options under the 2014 Plan. | |
| ● | On March 28, 2016, we furloughed all of our employees and independent contractors indefinitely and arranged with our Chief Executive Officer, David G. Watumull; our Chief Financial Officer, John B. Russell; and our Vice President, Operations, David M. Watumull, to continue their services for cash compensation equal to the minimum wage. In addition, each of the directors agreed, effective April 1, 2016, to suspend any additional equity compensation, until otherwise agreed by the Company. | |
| ● | On June 3, 2016, the compensation arrangement of David M. Watumull was amended so that, effective May 30, 2016, he would receive bi-weekly compensation equal to $3,269 and the compensation arrangement of Timothy J. King was amended so that, effective May 30, 2016, he would receive bi-weekly compensation equal to $1,635. | |
| ● | On September 6, 2016, the compensation arrangements of certain officers were amended so that effective September 8, 2016, (i) David G. Watumull would receive bi-weekly compensation equal to $4,327, (ii) Gilbert M. Rishton would receive bi-weekly compensation equal to $1,923, and (iii) Timothy J. King would receive bi-weekly compensation equal to $3,269. | |
| ● | On August 31, 2017, the compensation arrangements of certain officers were amended so that effective September 1, 2017, (i) David G. Watumull would receive bi-weekly compensation equal to $7,212, (ii) David M. Watumull would receive bi-weekly compensation equal to $5,769, (iii) Gilbert M. Rishton would receive bi-weekly compensation equal to $4,904, and (iv) Timothy J. King would receive bi-weekly compensation equal to $4,904. |
On July 30, 2013, we entered into a service agreement with JBR Business Solutions, LLC, under which John B. Russell agreed to serve as our Chief Financial Officer, and under which Mr. Russell would be paid an aggregate of $7,000 a month. Mr. Russell is the Managing Partner of JBR Business Solutions, LLC. To conserve cash resources while seeking additional financing, we and Mr. Russell, agreed to reduce cash compensation effective January 15, 2015. On June 30, 2015, the compensation arrangement was amended so that, effective after June 30, 2015, we had the right to pay up to 50% of any compensation due during any calendar quarter that was not paid in cash in the form of shares of our common stock or non-qualified stock options under the 2014 Plan. On March 28, 2016, Mr. Russell was furloughed and agreed to continue service as Chief Financial Officer for cash compensation equal to the minimum wage. On September 6, 2016, the compensation arrangement was amended so that effective September 30, 2016, he would receive monthly compensation of $3,500. On August 31, 2017, the compensation arrangement was amended so that effective September 1, 2017, Mr. Russell would receive monthly compensation of $5,250.
| 67 |
Director Compensation
On June 30, 2015, we entered into an agreement with George W. Bickerstaff, III and Terence A. Kelly, Ph.D. that provided for the annual compensation of each independent director equal to $100,000, payable quarterly in arrears in the form of a grant of shares of our common stock or non-qualified stock options to purchase shares of our common stock under the 2014 Plan.
Effective April 1, 2016, the independent directors of the Company agreed to suspend any additional equity compensation, until otherwise agreed by the Company.
On September 6, 2016, the compensation arrangements of the independent directors of the Company were amended so that effective September 30, 2016, they would each receive quarterly equity compensation of $12,500 in arrears in the form of a grant of shares of our common stock or non-qualified stock options to purchase shares of our common stock under the 2014 Plan based on the higher of the then current market price or $30.00 per share, with such compensation prorated for one of three months for the quarter ended September 30, 2016.
On January 4, 2017, our Board of Directors elected Michele Galen to serve as an independent director until our next annual meeting of stockholders with quarterly equity compensation of $12,500 in arrears in the form of a grant of shares of our common stock or non-qualified stock options to purchase shares of our common stock under the 2014 Plan based on the higher of the then current market price or $30.00 per share.
On August 31, 2017, the compensation arrangements of the independent directors of the Company were amended so that effective September 1, 2017, they would each receive quarterly equity compensation of $18,750 in arrears in the form of a grant of shares of our common stock or non-qualified stock options to purchase shares of our common stock under the 2014 Plan based on the higher of the then current market price or $30.00 per share. In 2018, independent director compensation was updated such that up to one-third could be payable in cash at the election of the director.
On June 1, 2018, our Board of Directors elected Makarand Jawadekar, Ph.D. and Elona Kogan to serve as independent directors until our next annual meeting of stockholders with quarterly compensation of $18,750 in arrears in the form of a grant of shares of our common stock or non-qualified stock options to purchase shares of our common stock under the 2014 Plan based on the higher of the then current market price or $30.00 per share, with up to one-third payable in cash at the election of the director. Dr. Jawadekar’s and Ms. Kogan’s compensation for the second quarter of 2018 was prorated to $6,250.
2014 Equity Compensation Plan
Our 2014 Plan is administered by our compensation committee. The purpose of the 2014 Plan is to provide financial incentives for selected directors, employees, advisers, and consultants of Cardax and/or its subsidiaries, thereby promoting the long-term growth and financial success of the Company. The issuance of awards under the 2014 Plan is at the discretion of our compensation committee, which has the authority to determine the persons to whom any awards shall be granted and the terms, conditions, and restrictions applicable to any award. Under the 2014 Plan, we may grant equity-based incentive awards, including options, restricted stock, and other stock-based awards, to any directors, employees, advisers, and consultants that provide services to us or any of our subsidiaries. The 2014 Plan also permits us to amend the terms of previously granted options or other awards. An aggregate of 279,101 shares of our common stock are presently reserved for issuance under the 2014 Plan, which is subject to adjustment as described in such plan. On December 4, 2018, our stockholders and our Board of Directors authorized the annual increase of the Plan Shares on January 1st of each year, at the discretion of our Board of Directors, by up to such number of shares that is equal to four percent (4%) of the shares of our common stock issued and outstanding as of December 31st of the previous calendar year; accordingly, effective as of January 1, 2020, the Plan Shares were increased by 27,000 shares. As of the date of this report, there are 39,191 shares of our common stock available for future awards under the 2014 Plan.
| 68 |
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Securities Authorized for Issuance under Equity Compensation Plans
The information required by Item 201(d) of Regulation S-K regarding our 2014 Plan is outlined above in Item 5 of this Annual Report on Form 10-K.
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth information regarding the ownership of our common stock as of March 30, 2020, for:
| ● | each director; | |
| ● | each person known by us to own beneficially 5% or more of our common stock; | |
| ● | each officer named in the summary compensation table elsewhere in this report; and | |
| ● | all directors and executive officers as a group. |
The amounts and percentages of our common stock beneficially owned are reported on the basis of regulations of the SEC governing the determination of beneficial ownership of securities. Under the rules of the SEC, a person is deemed to be a “beneficial owner” of a security if that person has or shares “voting power,” which includes the power to vote or to direct the voting of such security, or “investment power,” which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which that person has the right to acquire beneficial ownership within 60 days. Under these rules more than one person may be deemed a beneficial owner of the same securities and a person may be deemed to be a beneficial owner of securities as to which such person has no economic interest.
Unless otherwise indicated below, to our knowledge each beneficial owner named in the table has sole voting and sole investment power with respect to all shares beneficially owned, subject to community property laws where applicable.
| Name | Amount of Beneficial Ownership of Common Stock | Percent of Common Stock(1) | ||||||
| Directors and Executive Officers | ||||||||
| George W. Bickerstaff, III(2) | 23,740 (3) | 3.1 | % | |||||
| Terence A. Kelly, Ph.D.(4) | 9,010 (5) | 1.2 | ||||||
| Michele Galen(6) | 5,673 (7) | * | ||||||
| Makarand Jawadekar, Ph.D.(8) | 6,053 (9) | * | ||||||
| Elona Kogan(10) | 3,448 (11) | * | ||||||
| David G. Watumull(12) | 56,761 (13) | 7.0 | % | |||||
| David M. Watumull(14) | 18,074 (15) | 2.3 | % | |||||
| John B. Russell(16) | 1,662 (17) | * % | ||||||
| All directors and executive officers as a group (6 persons) | 124,421 | 14.9 | % | |||||
| Beneficial Owner of 5% or more | ||||||||
| Eric J. Pearson and Lianne L. Pearson(18) | 221,462 (19) | 25.7 | % | |||||
| James K. Schuler(20) | 325,165 (21) | 31.0 | % | |||||
| * | Represents beneficial ownership of our common stock of less than 1%. |
| 69 |
| (1) | Based on 693,419 shares of our common stock issued and outstanding as of March 30, 2020. |
| (2) | The address of Mr. George W. Bickerstaff, III is c/o Cardax, Inc., 2800 Woodlawn Drive, Honolulu, Hawaii 96822. Mr. Bickerstaff is the current Chairman of our Board of Directors. |
| (3) | Represents 23,740 shares of our common stock. |
| (4) | The address of Dr. Terence A. Kelly is c/o Cardax, Inc., 2800 Woodlawn Drive, Honolulu, Hawaii 96822. Dr. Kelly is a member of our Board of Directors. |
| (5) | Represents (a) 5,562 shares of our common stock, (b) 2,084 shares of our common stock issuable upon exercise by Dr. Kelly of options that are presently exercisable, at an exercise price of $12.00 per share, (c) 556 shares of our common stock issuable upon exercise by Dr. Kelly of options that are presently exercisable, at an exercise price of $30.00 per share, (d) 391 shares of our common stock issuable upon exercise by Dr. Kelly of options that are presently exercisable, at an exercise price of $37.00 per share, and (e) 417 shares of our common stock issuable upon exercise by Dr. Kelly of options that are presently exercisable, at an exercise price of $40.00 per share. |
| (6) | The address of Ms. Michele Galen is c/o Cardax, Inc., 2800 Woodlawn Drive, Honolulu, Hawaii 96822. Ms. Galen is a member of our Board of Directors. |
| (7) | Represents 5,673 shares of our common stock. |
| (8) | The address of Dr. Makarand Jawadekar, Ph.D. is c/o Cardax, Inc., 2800 Woodlawn Drive, Honolulu, Hawaii 96822. Dr. Jawadekar is a member of our Board of Directors. |
| (9) | Represents (a) 3,448 shares of our common stock, (b) 477 shares of our common stock issuable upon exercise of options that are presently exercisable, at an exercise price of $64.00 per share, which Dr. Jawadekar may be deemed to beneficially own as the principal of Melinda Consulting, LLC, (c) 577 shares of our common stock issuable upon exercise of options that are presently exercisable, at an exercise price of $40.00 per share, which Dr. Jawadekar may be deemed to beneficially own as the principal of Melinda Consulting, LLC, (d) 130 shares of our common stock issuable upon exercise of options that are presently exercisable, at an exercise price of $98.00 per share, which Dr. Jawadekar may be deemed to beneficially own as the principal of Melinda Consulting, LLC, (e) 171 shares of our common stock issuable upon exercise of options that are presently exercisable, at an exercise price of $54.00 per share, which Dr. Jawadekar may be deemed to beneficially own as the principal of Melinda Consulting, LLC, and (f) 1,250 shares of our common stock issuable upon exercise of options that are presently exercisable, at an exercise price of $12.00 per share, which Dr. Jawadekar may be deemed to beneficially own as the principal of Melinda Consulting, LLC. |
| (10) | The address of Ms. Elona Kogan is c/o Cardax, Inc., 2800 Woodlawn Drive, Honolulu, Hawaii 96822. Ms. Kogan is a member of our Board of Directors. |
| (11) | Represents 3,448 shares of our common stock. |
| 70 |
| (12) | The address of Mr. David G. Watumull is c/o Cardax, Inc., 2800 Woodlawn Drive, Honolulu, Hawaii 96822. Mr. David G. Watumull is our President, CEO, and a member of our Board of Directors. |
| (13) | Represents (a) 8,754 shares of our common stock issuable upon exercise by Mr. David G. Watumull of options that are presently exercisable, at an exercise price of $31.00 per share, (b) 24,710 shares of our common stock issuable upon exercise by Mr. David G. Watumull of options that are presently exercisable, at an exercise price of $125.00 per share, (c) 2,343 shares of our common stock issuable upon exercise by Mr. David G. Watumull of options that are presently exercisable, at an exercise price of $64.00 per share, (d) 1,954 shares of our common stock issuable upon exercise by Mr. David G. Watumull of options that are presently exercisable, at an exercise price of $40.00 per share, (e) 448 shares of our common stock issuable upon exercise by Mr. David G. Watumull of options that are presently exercisable, at an exercise price of $98.00 per share, (f) 689 shares of our common stock issuable upon exercise by Mr. David G. Watumull of options that are presently exercisable, at an exercise price of $54.00 per share, (g) 3,872 shares of our common stock issuable upon exercise by Mr. David G. Watumull of options that are presently exercisable, at an exercise price of $12.00 per share, (h) 1,991 shares of our common stock issued in the Holdings Merger, which Mr. Watumull may be deemed to beneficially own as the Trustee of the David G. Watumull Revocable Living Trust, (i) 1,750 shares of our common stock issued in the 2016/2017 Unit Offering, which Mr. Watumull may be deemed to beneficially own as the Trustee of the David G. Watumull Revocable Living Trust, (j) 1,750 shares of our common stock issuable upon exercise of a warrant issued in the 2016/2017 Unit Offering at an exercise price of $16.00 per share, which Mr. Watumull may be deemed to beneficially own as the Trustee of the David G. Watumull Revocable Living Trust, (k) 1,750 shares of our common stock issuable upon exercise of a warrant issued in the 2016/2017 Unit Offering at an exercise price of $24.00 per share, which Mr. Watumull may be deemed to beneficially own as the Trustee of the David G. Watumull Revocable Living Trust, (l) 1,750 shares of our common stock issuable upon exercise of a warrant issued in the 2016/2017 Unit Offering at an exercise price of $32.00 per share, which Mr. Watumull may be deemed to beneficially own as the Trustee of the David G. Watumull Revocable Living Trust, and (m) 5,000 shares of our common stock issuable upon conversion of a convertible note at an exercise price of $20.00 per share. |
| (14) | The address of Mr. David M. Watumull is c/o Cardax, Inc., 2800 Woodlawn Drive, Honolulu, Hawaii 96822. Mr. David M. Watumull is our Chief Operating Officer. |
| (15) | Represents (a) 226 shares of our common stock issuable upon exercise by Mr. David M. Watumull of options that are presently exercisable, at an exercise price of $31.00 per share, (b) 11,943 shares of our common stock issuable upon exercise by Mr. David M. Watumull of options that are presently exercisable, at an exercise price of $125.00 per share, (c) 805 shares of our common stock issuable upon exercise by Mr. David M. Watumull of options that are presently exercisable, at an exercise price of $64.00 per share, (d) 1,425 shares of our common stock issuable upon exercise by Mr. David M. Watumull of options that are presently exercisable, at an exercise price of $40.00 per share, (e) 339 shares of our common stock issuable upon exercise by Mr. David M. Watumull of options that are presently exercisable, at an exercise price of $98.00 per share, (f) 521 shares of our common stock issuable upon exercise by Mr. David M. Watumull of options that are presently exercisable, at an exercise price of $54.00 per share, and (g) 2,815 shares of our common stock issuable upon exercise by Mr. David M. Watumull of options that are presently exercisable, at an exercise price of $12.00 per share. |
| (16) | The address of Mr. John B. Russell is c/o Cardax, Inc., 2800 Woodlawn Drive, Honolulu, Hawaii 96822. Mr. Russell is our Chief Financial Officer. |
| (17) | Represents (a) 300 shares of our common stock issuable upon exercise of options that are presently exercisable, at an exercise price of $64.00 per share, which Mr. Russell may be deemed to beneficially own as the Managing Partner of JBR Business Solutions, LLC, (b) 313 shares of our common stock issuable upon exercise of options that are presently exercisable, at an exercise price of $40.00 per share, which Mr. Russell may be deemed to beneficially own as the Managing Partner of JBR Business Solutions, LLC, (c) 95 shares of our common stock issuable upon exercise of options that are presently exercisable, at an exercise price of $98.00 per share, which Mr. Russell may be deemed to beneficially own as the Managing Partner of JBR Business Solutions, LLC, (d) 125 shares of our common stock issuable upon exercise of options that are presently exercisable, at an exercise price of $54.00 per share, which Mr. Russell may be deemed to beneficially own as the Managing Partner of JBR Business Solutions, LLC, and (e) 829 shares of our common stock issuable upon exercise of options that are presently exercisable, at an exercise price of $12.00 per share, which Mr. Russell may be deemed to beneficially own as the Managing Partner of JBR Business Solutions, LLC. |
| 71 |
| (18) | The address of Dr. Eric J. Pearson and Mrs. Lianne L. Pearson is 814 Mokulua Drive, Kailua, Hawaii 96734. Dr. and Mrs. Pearson do not have any position, office, contractual relationship, or other understanding with the Company regarding the management or control of the Company and accordingly, we have determined that such stockholder is not an affiliate of Cardax. |
| (19) | Represents (a) 1,042 shares of our common stock issued in the 2017 Unit Offering, (b) 18,834 shares of our common stock issued in the 2017 Unit Offering, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Eric J Pearson DVM 401(k) Profit Sharing Plan FBO Eric J Pearson, (c) 20,151 shares of our common stock issued in the 2017 Unit Offering, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Eric J Pearson DVM 401(k) Profit Sharing Plan FBO Lianne Pearson, (d) 4,845 shares of our common stock issued in the 2017 Unit Offering, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Sunwest Trust FBO Lianne L. Pearson Roth IRA, (e) 6,172 shares of our common stock issued in the 2017 Unit Offering, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Sunwest Trust FBO Eric J. Pearson Roth IRA, (f) 2,000 shares of our common stock issued in the 2017 Unit Offering, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Sunwest Trust FBO Eric J. Pearson Roth IRA, (g) 38,814 shares of our common stock issued in the 2017 Unit Offering, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Eric J Pearson DVM 401(k) Profit Sharing Plan FBO Eric J Pearson, (h) 10,704 shares of our common stock issued in the 2017 Unit Offering, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Eric J Pearson DVM 401(k) Profit Sharing Plan FBO Lianne Pearson, (i) 333 shares of our common stock issued in the 2017(3) Unit Offering, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Sunwest Trust as Custodian for Lianne Pearson Roth IRA, (j) 15,500 shares of our common stock issued in the 2018 Warrant Exchange Offering, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Sunwest Trust FBO Eric J. Pearson Roth IRA, (k) 172 shares of our common stock issued in the 2018 Warrant Exchange Offering, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Sunwest Trust FBO Lianne L. Pearson Roth IRA, (l) 1,042 shares of our common stock issuable upon exercise of a warrant issued in the 2017 Unit Offering at an exercise price of $24.00 per share, (m) 18,834 shares of our common stock issuable upon exercise of a warrant issued in the 2017 Unit Offering at an exercise price of $24.00 per share, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Eric J Pearson DVM 401(k) Profit Sharing Plan FBO Eric J Pearson, (n) 20,151 shares of our common stock issuable upon exercise of a warrant issued in the 2017 Unit Offering at an exercise price of $24.00 per share, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Eric J Pearson DVM 401(k) Profit Sharing Plan FBO Lianne Pearson, (o) 4,845 shares of our common stock issuable upon exercise of a warrant issued in the 2017 Unit Offering at an exercise price of $24.00 per share, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Sunwest Trust FBO Lianne L. Pearson Roth IRA, (p) 6,172 shares of our common stock issuable upon exercise of a warrant issued in the 2017 Unit Offering at an exercise price of $24.00 per share, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Sunwest Trust FBO Eric J. Pearson Roth IRA, (q) 2,000 shares of our common stock issuable upon exercise of a warrant issued in the 2017 Unit Offering at an exercise price of $24.00 per share, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Sunwest Trust FBO Eric J. Pearson Roth IRA, (r) 38,814 shares of our common stock issuable upon exercise of a warrant issued in the 2017 Unit Offering at an exercise price of $24.00 per share, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Eric J Pearson DVM 401(k) Profit Sharing Plan FBO Eric J Pearson, (s) 10,704 shares of our common stock issuable upon exercise of a warrant issued in the 2017 Unit Offering at an exercise price of $24.00 per share, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Eric J Pearson DVM 401(k) Profit Sharing Plan FBO Lianne Pearson, and (t) 333 shares of our common stock issuable upon exercise of a warrant issued in the 2017(3) Unit Offering at an exercise price of $60.00 per share, which the Pearson’s may be deemed to beneficially own as the beneficiaries of the Sunwest Trust as Custodian for Lianne Pearson Roth IRA. |
| 72 |
| (20) | The address of Mr. James K. Schuler is 828 Fort Street Mall, Honolulu, HI 96813. Mr. Schuler does not have any position, office, contractual relationship, or other understanding with the Company regarding the management or control of the Company and accordingly, we have determined that such stockholder is not an affiliate of Cardax. |
| (21) | Represents (a) 23,373 shares of our common stock which Mr. Schuler may be deemed to beneficially own as the President of JKS Partners, L.P., (b) 9,803 shares of our common stock which Mr. Schuler may be deemed to beneficially own as the President of The Schuler Family Foundation, (c) 2,021 shares of our common stock which Mr. Schuler may be deemed to beneficially own as the Trustee of the James K. Schuler Revocable Living Trust, (d) 13,336 shares of our common stock issuable upon exercise of warrants issued in the 2015 Unit Offering at an exercise price of $20.00 per share, which Mr. Schuler may be deemed to beneficially own as the President of JKS Partners, L.P., (e) 5,000 shares of our common stock issuable upon exercise of warrants issued in the 2015 Unit Offering at an exercise price of $33.34 per share, which Mr. Schuler may be deemed to beneficially own as the President of JKS Partners, L.P., (f) 6,250 shares of our common stock issuable upon exercise of a warrant issued in the 2016-2017 Unit Offering at an exercise price of $16.00 per share, which Mr. Schuler may be deemed to beneficially own as the President of JKS Partners, L.P., (g) 6,250 shares of our common stock issuable upon exercise of a warrant issued in the 2016-2017 Unit Offering at an exercise price of $24.00 per share, which Mr. Schuler may be deemed to beneficially own as the President of JKS Partners, L.P., (h) 6,250 shares of our common stock issuable upon exercise of a warrant issued in the 2016-2017 Unit Offering at an exercise price of $32.00 per share, which Mr. Schuler may be deemed to beneficially own as the President of JKS Partners, L.P., (i) 1,042 shares of our common stock issuable upon exercise of a warrant issued in the 2017 Unit Offering at an exercise price of $24.00 per share, which Mr. Schuler may be deemed to beneficially own as the President of JKS Partners, L.P., (j) 510 shares of our common stock issuable upon exercise of a warrant issued in the Holdings Merger at an exercise price of $196.11 per share, which Mr. Schuler may be deemed to beneficially own as the President of The Schuler Family Foundation, (k) 190,918 shares of our common stock issuable upon conversion of a convertible note at an exercise price of $4.27 per share, which Mr. Schuler may be deemed to beneficially own as the President of JKS Partners, L.P., (l) 7,500 shares of our common stock issuable upon exercise of a warrant issued in connection with a convertible note at an exercise price of $4.27 per share, which Mr. Schuler may be deemed to beneficially own as the President of JKS Partners, L.P., (m) 50,912 shares of our common stock issuable upon conversion of a convertible note at an exercise price of $4.27 per share, which Mr. Schuler may be deemed to beneficially own as the President of JKS Partners, L.P., and (n) 2,000 shares of our common stock issuable upon exercise of a warrant issued in connection with a convertible note at an exercise price of $4.27 per share, which Mr. Schuler may be deemed to beneficially own as the President of JKS Partners, L.P. |
| 73 |
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Transactions with Related Persons
On October 16, 2017, we engaged M.M. Dillon & Co., to serve as the Financial Advisor for the Company in connection with an exchange offer and related transactions. George W. Bickerstaff, III, the Chairman of our Board of Directors, is currently a Managing Director of M.M. Dillon & Co., and as such, abstained from voting on the engagement of M.M. Dillon & Co. On May 2, 2018, the terms of M.M. Dillon & Co.’s engagement were modified, and such modifications were approved by our Board of Directors (with Mr. Bickerstaff abstaining) on April 30, 2018. We raised $1.44 million in gross proceeds in the exchange offer, which closed on July 27, 2018. As the Financial Advisor in connection with the exchange offer, M.M. Dillon & Co. was paid a cash fee of 3.5% of the gross proceeds from the exchange offer, or $50,402, and a 5-year common stock purchase warrant with a fair market value equal to 3.5% of the gross proceeds from the exchange offer, or $50,402.
On June 26, 2019, we and our Chief Executive Officer, as a lender to the Company, entered into a promissory note payable in the amount of $75,000. This note accrues interest at the rate of 4.5% per annum and has been amended so that it matures on June 30, 2020.
On November 15, 2019, we and our Chief Executive Officer, as a lender to the Company, entered into a convertible note payable in the amount of $100,000. This note accrues interest payable monthly at the rate of 14% per annum and matures on June 30, 2020. This note and accrued interest thereon may convert into shares of our common stock at $20.00 per share any time at the holder’s option. If this note, or any portion thereof, has not been repaid or converted in full on or prior to the maturity date, then repayment of the unpaid principal balance plus any accrued and unpaid interest thereon, shall be amortized over the following thirty-six (36) months. We have the right to prepay this note without penalty or premium.
Other than compensation arrangements with directors and executive officers, which are described under “Executive Compensation— Employment and Consulting Agreements”, and except as described above, we have no other related-party transactions that are subject to disclosure.
Director Independence
George W. Bickerstaff, III, Michele Galen, Terence A. Kelly, Ph.D., Makarand Jawadekar, Ph.D., and Elona Kogan are our independent directors. Because our common stock is not currently listed on a national securities exchange, we have used the definition of “independence” of The NASDAQ Stock Market to make this determination. NASDAQ Listing Rule 5605(a)(2) provides that an “independent director” is a person other than an officer or employee of the Company or any other individual having a relationship that, in the opinion of the Company’s Board, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. The NASDAQ listing rules provide that a director cannot be considered independent if:
| ● | the director is, or at any time during the past three years was, an employee of the Company; | |
| ● | the director or a family member of the director accepted any compensation from the Company in excess of $120,000 during any period of 12 consecutive months within the three years preceding the independence determination (subject to certain exclusions, including, among other things, compensation for board or board committee service); | |
| ● | a family member of the director is, or at any time during the past three years was, an executive officer of the Company; | |
| ● | the director or a family member of the director is a partner in, controlling stockholder of, or an executive officer of an entity to which the Company made, or from which the Company received, payments in the current or any of the past three fiscal years that exceed 5% of the recipient’s consolidated gross revenue for that year or $200,000, whichever is greater (subject to certain exclusions); | |
| ● | the director or a family member of the director is employed as an executive officer of an entity where, at any time during the past three years, any of the executive officers of the Company served on the compensation committee of such other entity; or | |
| ● | the director or a family member of the director is a current partner of the Company’s outside auditor, or at any time during the past three years was a partner or employee of the Company’s outside auditor, and who worked on the Company’s audit. |
| 74 |
Item 14. Principal Accounting Fees and Services.
We engaged KBL, LLP as our independent registered public accounting firm for the years ended December 31, 2019 and 2018. The table below sets forth the aggregate fees billed for fiscal years ended December 31, 2019 and 2018, for professional services rendered by KBL, LLP, for the audit of our annual consolidated financial statements in our annual reports on Form 10-K, review of the consolidated financial statements included in our quarterly reports on Form 10-Q, and services that are normally provided in connection with statutory and regulatory filings or engagements.
Fiscal Year Ended December 31, 2019 | Fiscal Year Ended December 31, 2018 | |||||||
| Audit Fees(1) | $ | 80,000 | * | $ | 72,000 | * | ||
| Audit-Related Fees(2) | $ | - | $ | - | ||||
| Tax Fees(3) | $ | - | $ | - | ||||
| All Other Fees(4) | $ | - | $ | - | ||||
| Total | $ | 80,000 | $ | 72,000 | ||||
| * | The amounts of audit fees disclosed for our fiscal years ended December 31, 2019 and 2018, represent the aggregate audit fees billed for 2019 and 2018, respectively. The amount billed for 2019 includes fees incurred in connection with the audit of our financial statements for the fiscal year ended December 31, 2018 and the review of our interim financial statements in 2019. The amount billed for 2018 includes fees incurred in connection with the audit of our financial statements for the fiscal year ended December 31, 2017 and the review of our interim financial statements in 2018. |
| (1) | Audit fees consist of fees incurred for professional services rendered for the audit of our financial statements, for reviews of our interim financial statements included in our quarterly reports on Form 10-Q and for services that are normally provided in connection with statutory or regulatory filings or engagements. |
| (2) | Audit-related fees consist of fees billed for professional services that are reasonably related to the performance of the audit or review of our financial statements, but are not reported “Audit Fees.” |
| (3) | Tax fees consist of fees billed for professional services relating to tax compliance, tax advice, and tax planning. |
| (4) | All other fees consist of fees billed for products and services provided by our principal accountants, other than for products and services reported above. |
Audit Committee’s Pre-Approval Policies
Our audit committee is responsible for, among other things, the selection, appointment, retention and dismissal of our independent auditors. Additionally, our audit committee pre-approves the retention of our independent auditors for any non-audit services, and the funding for payment of compensation to our independent auditors for both audit and non-audit services.
Audit Hours Incurred
Less than fifty percent of the hours expended on our principal accountant’s engagement to audit our financial statements for the most recent fiscal year were attributed to work performed by persons other than our principal accountant’s full-time, permanent employees.
| 75 |
Item 15. Exhibits, Financial Statement Schedules.
(a) Financial Statements
| Page | |
| Report of Independent Registered Public Accounting Firm | F-2 |
| Consolidated financial statements: | |
| Consolidated balance sheets | F-3 |
| Consolidated statements of operations | F-4 |
| Consolidated statement of changes in stockholders’ deficit | F-5 |
| Consolidated statements of cash flows | F-6 |
| Notes to the consolidated financial statements | F-7 |
(b) Financial Statement Schedules
All consolidated financial statement schedules are included in the footnotes to the financial statements, are inapplicable, or otherwise not required.
| 76 |
(c) Exhibits
| 77 |
| 78 |
| 79 |
| 101.INS* | XBRL Instance Document | |
| 101.SCH* | XBRL Taxonomy Extension Schema Document | |
| 101.CAL* | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF* | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB* | XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE* | XBRL Taxonomy Extension Presentation Linkbase Document |
| * | Filed herewith. |
| (1) | Filed as an exhibit to the Current Report on Form 8-K of the Company filed November 29, 2013. |
| (2) | Filed as an exhibit to the Current Report on Form 8-K of the Company filed January 14, 2014. |
| (3) | Filed as an exhibit to the Current Report on Form 8-K of the Company filed February 10, 2014. |
| (4) | Filed as an exhibit to the Current Report on Form 8-K of the Company filed November 24, 2015. |
| (5) | Filed as an exhibit to the Current Report on Form 8-K of the Company filed January 14, 2020. |
| (6) | Filed as an exhibit to the Registration Statement on Form S-1 of the Company dated August 14, 2019. |
| (7) | Filed as an exhibit to the Amendment No. 1 to Registration Statement on Form S-1 of the Company dated September 2, 2014. |
| (8) | Filed as an exhibit to the Current Report on Form 8-K of the Company filed March 9, 2015. |
| (9) | Filed as an exhibit to the Current Report on Form 8-K of the Company filed July 7, 2015. |
| (10) | Filed as an exhibit to the Quarterly Report on Form 10-Q of the Company filed May 13, 2016. |
| (11) | Filed as an exhibit to the Current Report on Form 8-K of the Company filed July 18, 2016. |
| (12) | Filed as an exhibit to the Annual Report on Form 10-K of the Company filed March 31, 2017. |
| (13) | Filed as an exhibit to the Quarterly Report on Form 10-Q of the Company filed November 14, 2017. |
| (14) | Filed as an exhibit to the Current Report on Form 8-K of the Company filed October 20, 2017. |
| (15) | Filed as an exhibit to the Quarterly Report on Form 10-Q of the Company filed July 25, 2019. |
| (16) | Filed as an exhibit to the Amendment No. 2 of the Registration Statement on Form S-1 of the Company dated November 22, 2019. |
| (17) | Filed as an exhibit to the Current Report on Form 8-K of the Company filed January 27, 2020. |
| (18) | Filed as an exhibit to the Current Report on Form 8-K of the Company filed March 20, 2020. |
| 80 |
SIGNATURES
Pursuant to the requirements of Section 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: March 30, 2020
| CARDAX, INC. | ||
| By: | /s/ David G. Watumull | |
| Name: | David G. Watumull | |
| Title: | Chief Executive Officer and President | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ David G. Watumull | President, Chief Executive Officer, and Director | March 30, 2020 | ||
| David G. Watumull | ||||
| /s/ John B. Russell | Chief Financial Officer and Treasurer | March 30, 2020 | ||
| John B. Russell | ||||
| /s/ George W. Bickerstaff, III | Chairman | March 30, 2020 | ||
| George W. Bickerstaff, III | ||||
| /s/ Terence A. Kelly | Director | March 30, 2020 | ||
| Terence A. Kelly, Ph.D. | ||||
| /s/ Michele Galen | Director | March 30, 2020 | ||
| Michele Galen | ||||
| /s/ Makarand Jawadekar, Ph.D. | Director | March 30, 2020 | ||
| Makarand Jawadekar, Ph.D. | ||||
| /s/ Elona Kogan | Director | March 30, 2020 | ||
| Elona Kogan |
| 81 |
Consolidated Financial Statements
Cardax, Inc., and Subsidiary
December 31, 2019 and 2018
Contents
| Page | |
| CONSOLIDATED FINANCIAL STATEMENTS: | |
| Report of Independent Registered Public Accounting Firm | F-2 |
| Consolidated balance sheets | F-3 |
| Consolidated statements of operations | F-4 |
| Consolidated statement of changes in stockholders’ deficit | F-5 |
| Consolidated statements of cash flows | F-6 |
| Notes to the consolidated financial statements | F-7 |
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
of Cardax, Inc. and Subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Cardax, Inc. and Subsidiaries (the “Company”) as of December 31, 2019 and 2018, the related consolidated statements of operations, changes in stockholders’ deficit, and cash flows for the years then ended, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the consolidated financial position of the Company as of December 31, 2019 and 2018, and the results of its consolidated operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal controls over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Going Concern Consideration
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has sustained significant operating losses and needs to obtain additional financing to continue the services they provide. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ KBL, LLP
We have served as the Company’s auditor since 2013.
KBL, LLP
New York, NY
March 30, 2020
| F-2 |
CONSOLIDATED BALANCE SHEETS
As of December 31,
| 2019 | 2018 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash | $ | 19,303 | $ | 243,753 | ||||
| Accounts receivable | 205,768 | 157,082 | ||||||
| Inventories | 1,177,831 | 1,480,380 | ||||||
| Deposits and other assets | 2,066 | 119,066 | ||||||
| Prepaid expenses | 181,093 | 24,083 | ||||||
| Total current assets | 1,586,061 | 2,024,364 | ||||||
| INTANGIBLE ASSETS, net | 420,373 | 434,534 | ||||||
| RIGHT TO USE LEASED ASSETS | 12,488 | - | ||||||
| TOTAL ASSETS | $ | 2,018,922 | $ | 2,458,898 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accrued payroll and payroll related expenses, current portion | $ | 3,687,376 | $ | 3,428,011 | ||||
| Accounts payable and accrued expenses | 1,544,402 | 1,996,097 | ||||||
| Fees payable to directors | 418,546 | 418,546 | ||||||
| Accrued separation costs, current portion | 9,000 | 9,000 | ||||||
| Current portion of related party notes payable | 575,000 | - | ||||||
| Related party convertible notes payable | 651,721 | - | ||||||
| Convertible notes payable, net of discount | 358,289 | - | ||||||
| Employee settlement | 50,000 | 50,000 | ||||||
| Lease liability, current portion | 11,527 | - | ||||||
| Derivative liability on convertible note payable | 827,314 | - | ||||||
| Total current liabilities | 8,133,175 | 5,901,654 | ||||||
| NON-CURRENT LIABILITIES | ||||||||
| Related party notes payable, net of current portion | 1,000,000 | - | ||||||
| Accrued separation costs, less current portion | 83,635 | 92,635 | ||||||
| Lease liability, less current portion | 961 | - | ||||||
| Total non-current liabilities | 1,084,596 | 92,635 | ||||||
| COMMITMENTS AND CONTINGENCIES | - | - | ||||||
| Total liabilities | 9,217,771 | 5,994,289 | ||||||
| STOCKHOLDERS’ DEFICIT | ||||||||
| Preferred Stock - $0.001 par value; 50,000,000 shares authorized, 0 shares issued and outstanding as of December 31, 2019 and 2018, respectively | - | - | ||||||
| Common stock - $0.001 par value; 4,000,000 shares authorized, 687,564 and 669,967 shares issued and outstanding as of December 31, 2019 and 2018, respectively | 688 | 670 | ||||||
| Additional paid-in-capital | 59,836,818 | 58,407,257 | ||||||
| Accumulated deficit | (67,036,355 | ) | (61,943,318 | ) | ||||
| Total stockholders’ deficit | (7,198,849 | ) | (3,535,391 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | 2,018,922 | $ | 2,458,898 | ||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
| F-3 |
CONSOLIDATED STATEMENTS OF OPERATIONS
For the years ended December 31,
| 2019 | 2018 | |||||||
| REVENUES, net | $ | 710,949 | $ | 1,510,875 | ||||
| COST OF GOODS SOLD | 345,393 | 699,852 | ||||||
| GROSS PROFIT | 365,556 | 811,023 | ||||||
| OPERATING EXPENSES: | ||||||||
| Salaries and wages | 1,552,226 | 1,591,949 | ||||||
| Professional fees | 920,208 | 797,833 | ||||||
| Selling, general, and administrative expenses | 906,074 | 1,493,819 | ||||||
| Stock based compensation | 708,588 | 650,271 | ||||||
| Research and development | 315,994 | 269,077 | ||||||
| Depreciation and amortization | 39,569 | 30,569 | ||||||
| Total operating expenses | 4,442,659 | 4,833,518 | ||||||
| Loss from operations | (4,077,103 | ) | (4,022,495 | ) | ||||
| OTHER INCOME (EXPENSE): | ||||||||
| Interest income | - | 1,944 | ||||||
| Other income | - | 556 | ||||||
| Loss on abandonment of patents | (36,205 | ) | - | |||||
| Change in fair value of derivative liability | (356,314 | ) | - | |||||
| Interest expense | (623,415 | ) | (4,227 | ) | ||||
| Total other (expense) income, net | (1,015,934 | ) | (1,727 | ) | ||||
| Loss before the provision for income taxes | (5,093,037 | ) | (4,024,222 | ) | ||||
| PROVISION FOR INCOME TAXES | - | - | ||||||
| NET LOSS | $ | (5,093,037 | ) | $ | (4,024,222 | ) | ||
| NET LOSS PER SHARE | ||||||||
| Basic | $ | (7.49 | ) | $ | (6.32 | ) | ||
| Diluted | $ | (7.49 | ) | $ | (6.32 | ) | ||
| SHARES USED IN CALCULATION OF NET LOSS PER SHARE | ||||||||
| Basic | 680,152 | 637,028 | ||||||
| Diluted | 680,152 | 637,028 | ||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
| F-4 |
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS’ DEFICIT
Years ended December 31, 2019 and 2018
| Common Stock | Additional | Deferred | Accumulated | |||||||||||||||||||||
| Shares | Amount | Paid-In-Capital | Compensation | Deficit | Total | |||||||||||||||||||
| Balance at January 1, 2018 | 613,860 | $ | 614 | $ | 56,523,130 | $ | (10,125 | ) | $ | (57,919,096 | ) | $ | (1,405,477 | ) | ||||||||||
| Common stock grants to independent directors | 6,725 | 7 | 287,493 | - | - | 287,500 | ||||||||||||||||||
| Cardax 2018 Warrant Exchange Offering, net | 48,033 | 48 | 1,243,989 | - | - | 1,244,037 | ||||||||||||||||||
| Common stock grants to service providers | 563 | - | 22,500 | - | - | 22,500 | ||||||||||||||||||
| Stock option exercise | 786 | 1 | (1 | ) | - | - | - | |||||||||||||||||
| Deferred compensation | - | - | - | 10,125 | - | 10,125 | ||||||||||||||||||
| Stock based compensation - options | - | - | 330,146 | - | - | 330,146 | ||||||||||||||||||
| Net loss | - | - | - | - | (4,024,222 | ) | (4,024,222 | ) | ||||||||||||||||
| Balance at December 31, 2018 | 669,967 | 670 | 58,407,257 | - | (61,943,318 | ) | (3,535,391 | ) | ||||||||||||||||
| Common stock grants to independent directors | 11,054 | 11 | 349,989 | - | - | 350,000 | ||||||||||||||||||
| Restricted stock issuance | 8,169 | 8 | 244,992 | - | - | 245,000 | ||||||||||||||||||
| Common stock grants to service providers | 750 | 1 | 16,649 | - | - | 16,650 | ||||||||||||||||||
| Stock based compensation - options | - | - | 341,938 | - | - | 341,938 | ||||||||||||||||||
| Issuance of warrants attached to a convertible note | - | - | 125,545 | - | - | 125,545 | ||||||||||||||||||
| Beneficial conversion feature issued on convertible notes | - | - | 350,446 | - | - | 350,446 | ||||||||||||||||||
| Retirement of issued stock | (2,376 | ) | (2 | ) | 2 | - | - | - | ||||||||||||||||
| Net loss | - | - | - | - | (5,093,037 | ) | (5,093,037 | ) | ||||||||||||||||
| Balance at December 31, 2019 | 687,564 | $ | 688 | $ | 59,836,818 | $ | - | $ | (67,036,355 | ) | $ | (7,198,849 | ) | |||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
| F-5 |
CONSOLIDATED STATEMENTS OF CASH FLOWS
For the years ended December 31,
| 2019 | 2018 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (5,093,037 | ) | $ | (4,024,222 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 39,569 | 30,570 | ||||||
| Amortization of debt discount | 417,001 | - | ||||||
| Stock based compensation | 708,588 | 650,271 | ||||||
| Bad debt expense on note receivable and accrued interest | - | 89,933 | ||||||
| Loss on abandonment of patents | 36,205 | - | ||||||
| Change in fair value of derivative liability | 356,314 | - | ||||||
| Changes in assets and liabilities: | ||||||||
| Accounts receivable | (138,258 | ) | 181,960 | |||||
| Inventories | 302,549 | 97,736 | ||||||
| Deposits and other assets | 117,000 | (118,168 | ) | |||||
| Prepaid expenses | (157,010 | ) | (1,245 | ) | ||||
| Accrued payroll and payroll related expenses | 259,365 | 39,421 | ||||||
| Accounts payable and accrued expenses | (362,123 | ) | (146,784 | ) | ||||
| Accrued separation costs | (9,000 | ) | - | |||||
| Net cash used in operating activities | (3,522,837 | ) | (3,200,528 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Increase in intangible assets | (61,613 | ) | (36,593 | ) | ||||
| Net cash used in investing activities | (61,613 | ) | (36,593 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from the issuances of related party notes payable | 1,575,000 | - | ||||||
| Proceeds from the issuances of a related party convertible note payable | 1,050,000 | - | ||||||
| Proceeds from the issuances of convertible notes payable | 490,000 | - | ||||||
| Proceeds from the issuance of common stock | 245,000 | 1,244,037 | ||||||
| Net cash provided by financing activities | 3,360,000 | 1,244,037 | ||||||
| NET DECREASE IN CASH | (224,450 | ) | (1,993,084 | ) | ||||
| BEGINNING OF THE PERIOD | 243,753 | 2,236,837 | ||||||
| END OF THE PERIOD | $ | 19,303 | $ | 243,753 | ||||
| SUPPLEMENTAL DISCLOSURES: | ||||||||
| Cash paid for interest | $ | 137,688 | $ | 4,227 | ||||
| Cash paid for income taxes | $ | - | $ | - | ||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Discount recognized on notes payable at issuance | $ | 946,991 | $ | - | ||||
| Settlement of receivables with payables | $ | 89,572 | $ | 301,799 | ||||
| Right to use assets funded through leases | $ | 12,488 | $ | - | ||||
| Purchases of inventory in accounts payable | $ | - | $ | 1,237,691 | ||||
| Retirement of issued stock | $ | 2 | $ | - | ||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
| F-6 |
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS
NOTE 1 – COMPANY BACKGROUND
The Company’s predecessor, Cardax Pharmaceuticals, Inc. (“Holdings”), was incorporated in the State of Delaware on March 23, 2006.
Cardax, Inc. (the “Company”) (OTCQB:CDXI) is a development stage biopharmaceutical company primarily focused on the development of pharmaceuticals for chronic diseases driven by inflammation. The Company also has a commercial business unit that markets dietary supplements for inflammatory health. CDX-101, the Company’s astaxanthin pharmaceutical candidate, is being developed for cardiovascular inflammation and dyslipidemia, with a target initial indication of severe hypertriglyceridemia. CDX-301, the Company’s zeaxanthin pharmaceutical candidate, is being developed for macular degeneration, with a target initial indication of Stargardt disease. The Company’s pharmaceutical candidates are currently in pre-clinical development, including the planning of IND enabling studies. ZanthoSyn® is a physician recommended astaxanthin dietary supplement for inflammatory health. The Company sells ZanthoSyn® primarily through wholesale and e-commerce channels. The safety and efficacy of the Company’s products have not been directly evaluated in clinical trials or confirmed by the FDA.
Going concern matters
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. As shown in the accompanying consolidated financial statements, the Company incurred net losses of $5,093,037 and $4,024,222 for the years ended December 31, 2019 and 2018, respectively. The Company has incurred losses since inception resulting in an accumulated deficit of $67,036,355 as of December 31, 2019 and has had negative cash flows from operating activities since inception. The Company expects that its marketing program for ZanthoSyn® will continue to focus on outreach to physicians, healthcare professionals, retail personnel, and consumers, and anticipates further losses in the development of its consumer business. The Company also plans to advance the research and development of its pharmaceutical candidates and anticipates further losses in the development of its pharmaceutical business. The Company’s ability to access the capital markets is unknown during the coronavirus disease 2019 (“COVID-19”) pandemic, which may limit or prevent the funding of its operations and related obligations. As a result of these and other factors, management has determined there is substantial doubt about the Company’s ability to continue as a going concern.
The Company needs to raise additional capital to carry out its business plan. During the year ended December 31, 2019, the Company raised additional capital to carry out its business plan. As part of the Company’s efforts, it raised an additional $3,360,000 in gross proceeds through the issuance of $245,000 in shares of common stock and $3,115,000 in debt instruments. In accordance with U.S. GAAP, derivative liabilities of $827,314 and beneficial conversion features of $350,446 were recognized in connection with convertible notes outstanding as of December 31, 2019; however, these are non-cash amounts and do not directly impact our liquidity or capital needs.
The Company filed a registration statement on Form S-1 on August 14, 2019, as amended September 27, 2019 and November 22, 2019, for a proposed $15 million public offering of common stock and warrants; however, there can be no assurance that the proposed public offering will be consummated. In addition, the Company may need to continue to obtain short-term debt financing on terms and conditions that may not otherwise be acceptable.
As of the date hereof, the Company has outstanding promissory notes (including notes payable, convertible notes payable, and secured convertible notes payable) that are (i) due June 30, 2020 in the aggregate principal amount of $2,403,176, (ii) due September 16, 2020 in the aggregate principal amount of $500,000, and (iii) due January 11, 2022 in the aggregate principal amount of $1,000,000. The Company’s ability to repay any and all of these notes as they become due if not otherwise repaid or converted on or prior to the maturity dates described above is uncertain and will be based on the Company’s ability to raise additional capital, generate additional revenues, and/or modify the terms of such debt instruments to the extent necessary.
The Company’s continued ability to raise additional capital through future equity and debt securities issuances is unknown. Obtaining additional financing, the successful development of the Company’s contemplated plan of operations, and its transition, ultimately, to profitable operations are necessary for the Company to continue operations. The ability to successfully resolve these factors raises substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements of the Company do not include any adjustments that may result from the outcome of these uncertainties.
| F-7 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of presentation
The consolidated financial statements have been consistently prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”) and include the accounts of Cardax, Inc., and its wholly owned subsidiary, Cardax Pharma, Inc., and its predecessor, Cardax Pharmaceuticals, Inc., which was merged with and into Cardax, Inc. All significant intercompany balances and transactions have been eliminated in consolidation.
Use of estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in our consolidated financial statements and the accompanying notes. Estimates in these consolidated financial statements include asset valuations, estimates of future cash flows from and the economic useful lives of long-lived assets, valuations of derivative liabilities, warrants, beneficial conversion features, and stock compensation, certain accrued liabilities, income taxes and tax valuation allowances, and fair value estimates. Despite management’s intention to establish accurate estimates and reasonable assumptions, actual results could differ materially from these estimates and assumptions.
Cash
The Company considers all highly liquid investments with maturities of three months or less at the time of purchase to be cash equivalents. The Company held no cash equivalents as of December 31, 2019 and 2018.
The Company maintains cash deposit accounts at one financial institution. Accounts at this institution are insured by the Federal Deposit Insurance Corporation up to $250,000. The Company’s cash balance at times may exceed these limits. As of December 31, 2019 and 2018, the Company had $0 in excess of federally insured limits on deposit.
Accounts receivable
Accounts receivable of $205,768 and $157,082 as of December 31, 2019 and 2018, respectively, consists of amounts due from sales of dietary supplements.
It is the Company’s policy to provide for an allowance for doubtful collections based upon a review of outstanding receivables, historical collection information, and existing economic conditions. Normal receivables are due 60 days after the issuance of the invoice. Receivables past due more than 90 days are considered delinquent. Delinquent receivables are written off based on individual credit evaluation and specific circumstances of the customer. There was no allowance necessary as of December 31, 2019 and 2018.
| F-8 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Inventories
Inventories are stated at the lower of cost or net realizable value. Cost is determined using the average cost method. Net realizable value is the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. Inventory costs include third party costs for finished goods. The Company utilizes contract manufacturers and receives inventory in finished form.
The Company provides a reserve against inventory for known or expected inventory obsolescence. The reserve is determined by specific review of inventory items for product age and quality that may affect salability. There were no reserves necessary for inventory as of December 31, 2019 and 2018.
Notes payable
During the year ended December 31, 2019, the Company issued various notes payable to related and non-related parties. These notes payable included original issue discounts, conversion features, and beneficial conversion features.
Original issue discounts. The Company accounts for the original issue discounts in accordance with Accounting Standards Codification (“ASC”) No. 835-30, Interest and Imputation of Interest, which requires the Company to record the discount as a contra liability and amortize it over the term of the underlying note using the interest method.
Detachable warrants. The Company accounts for detachable warrants in accordance with ASC No. 470-20, Debt, which requires the Company to bifurcate and separately account for the detachable warrant as a separated debt instrument. The values are assigned to detachable warrant based on a relative fair allocation between the note, the warrants, and any other debt instrument issued with the note payable. The fair value used for the warrant in this allocation is calculated using the Black-Scholes valuation model.
Conversion features. The Company accounts for the fair value of the conversion feature in accordance with ASC 815-15, Derivatives and Hedging; Embedded Derivatives, which requires the Company to bifurcate and separately account for the conversion feature as an embedded derivative contained in the Company’s convertible note. The Company is required to carry the embedded derivative on its balance sheet at fair value. The initial value of the embedded derivative is accounted for as a discount to the convertible note and a derivative liability. The liability is required to be remeasured at each reporting date and the change in fair value is recognized as a component in the results of operations. The Company valued the embedded derivatives on the consolidated balance sheet at fair value using the Black-Scholes valuation model.
Beneficial conversion features. The Company accounts for beneficial conversion features in accordance with ASC No. 470-20, Debt, which requires the Company to recognize a discount and charge an amount to additional paid in capital equal to the intrinsic value of the beneficial conversion feature upon issuance.
Stock issuance costs
Stock issuance costs related to financing are accounted for as a reduction in stock proceeds in accordance with ASC No. 340-10, Other Assets and Deferred Costs. Such costs consist of underwriting and legal fees, as well as travel costs incurred. These costs totaled $139,163 as of December 31, 2019 and are being deferred as a component of prepaid expenses in the accompanying consolidated balance sheet until completion of the offering.
| F-9 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Revenue from contracts with customers
In May 2014, the Financial Accounting Standards Board (“FASB”) issued a new standard related to revenue recognition. Under the standard, revenue is recognized when a customer obtains control of promised goods or services in an amount that reflects the consideration the entity expects to receive in exchange for those goods or services. In addition, the standard requires disclosure of the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers.
The Company adopted this standard effective January 1, 2018, using the retrospective method. As there was no impact on contracts that were previously completed and no significant impact to contracts completed after adoption, there was no need to restate prior results from operations.
The Company recognizes revenues from its contracts with customers for its products through wholesale and e-commerce channels when goods and services have been identified, the payment terms agreed to, the contract has commercial substance, both parties have approved the contract, and it is probable that the Company will collect all substantial consideration.
The following table presents our revenues disaggregated by revenue source and geographical location. Sales and usage-based taxes are included as a component of revenues for the years ended December 31:
| Geographical area | Source | 2019 | 2018 | |||||||
| United States | Nutraceuticals | $ | 705,694 | $ | 1,494,462 | |||||
| Hong Kong | Nutraceuticals | $ | 5,255 | $ | 16,413 | |||||
Sales discounts, rebates, promotional amounts to vendors, and returns and allowances are recorded as a reduction to sales in the period in which sales are recorded. Sales discounts and other adjustments are recorded at the time of sale.
Cost of goods sold
Cost of goods sold is comprised of costs to manufacture or acquire products sold to customers, direct and indirect distribution costs, and other costs incurred in the sale of goods.
Shipping and handling costs
Shipping and handling costs are included in cost of goods sold. Shipping and handling costs were $19,999 and $21,603 for the years ended December 31, 2019 and 2018, respectively.
Sales and use tax
Revenues, as presented on the accompanying income statement, include taxes collected from customers and remitted to governmental authorities. Such taxes were $3,018 and $3,329 for the years ended December 31, 2019 and 2018, respectively.
| F-10 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Research and development
Research and development costs are expensed as incurred and consists primarily of manufacturing of products, third-party research, clinical studies, laboratory supplies, and scientific advisory boards. The focus of these costs is on the development of astaxanthin, zeaxanthin, and related compounds. For the years ended December 31, 2019 and 2018, research and development costs were $315,994 and $269,077, respectively.
Advertising
Advertising costs are expensed as incurred and are included as an element of sales and marketing costs in the accompanying consolidated statements of operations. For the years ended December 31, 2019 and 2018, advertising costs were $161,468 and $364,306, respectively.
Income taxes
The Company accounts for income taxes under an asset and liability approach. Deferred income taxes reflect the impact of temporary differences between assets and liabilities recognized for financial reporting purposes and the amounts recognized for income tax reporting purposes, net operating loss carryforwards, and other tax credits measured by applying currently enacted tax laws. A valuation allowance is provided when necessary to reduce deferred tax assets to an amount that is more likely than not to be realized.
The Company determines whether a tax position is more likely than not to be sustained upon examination, including resolution of any related appeals or litigation processes, based on the technical merits of the position. The Company uses a two-step approach to recognizing and measuring uncertain tax positions. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained upon tax authority examination, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount that is more than 50% likely of being realized upon ultimate settlement.
The Company files income tax returns in the United States (“U.S.”) Federal, the States of Hawaii, California, and New York, and New York City jurisdictions. Tax regulations within each jurisdiction are subject to the interpretation of the related tax laws and regulations and require significant judgment to apply. The federal, state, and city income tax returns of the Company are subject to examination by the IRS, state, and city taxing authorities, generally for three years after they were filed.
The Company did not recognize any tax liabilities for income taxes associated with unrecognized tax benefits as of December 31, 2019 and 2018. The Company’s policy is to include interest and penalties related to unrecognized tax benefits, if any, within the provision for income taxes in the consolidated statements of operations.
| F-11 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Fair value measurements
U.S. GAAP establishes a framework for measuring fair value. That framework provides a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements).
The three levels of the fair value hierarchy are described below:
| Level 1: | Inputs to the valuation methodology are unadjusted quoted prices for identical assets or liabilities in active markets that the Company has the ability to access. | |
| Level 2: | Inputs to the valuation methodology include: |
| ● | Quoted prices for similar assets or liabilities in active markets; | |
| ● | Quoted prices for identical or similar assets or liabilities in inactive markets; | |
| ● | Inputs other than quoted prices that are observable for the asset or liability; and | |
| ● | Inputs that are derived principally from or corroborated by observable market data by correlation or other means. |
If the asset or liability has a specified (contractual) term, the Level 2 input must be observable for substantially the full term of the asset or liability.
| Level 3: | Inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
The asset’s or liability’s fair value measurement level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. Valuation techniques used need to maximize the use of observable inputs and minimize the use of unobservable inputs.
| F-12 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Stock based compensation
The Company accounts for stock-based compensation costs under the provisions of FASB’s Accounting Standards Codification ASC No. 718, Compensation—Stock Compensation and ASC No. 505, Equity, which requires the measurement and recognition of compensation expense related to the fair value of stock based compensation awards that are ultimately expected to vest. Stock based compensation expense recognized includes the compensation cost for all stock-based payments granted to employees, officers, directors, and consultants based on the grant date fair value estimated. These standards also apply to awards modified, repurchased, or canceled during the periods reported.
Basic and diluted net loss per share
Basic earnings per common share is calculated by dividing net loss for the year by the weighted average number of common shares outstanding during the year. Diluted earnings per common share is calculated by dividing net loss for the year by the sum of the weighted average number of common shares outstanding during the year plus the number of potentially dilutive common shares (“dilutive securities”) that were outstanding during the year. Dilutive securities include options granted pursuant to the Company’s stock option plans, and warrants issued to non-employees. Potentially dilutive securities are excluded from the computation of earnings per share in periods in which a net loss is reported, as their effect would be antidilutive.
Recently issued accounting pronouncements
In August 2018, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2018-13, Fair Value Measurement. This ASU modifies the disclosure requirements on fair value measurements in Topic 820, Fair Value Measurement, based on the concepts in the FASB’s Concepts Statement, including the consideration of costs and benefits. The guidance in ASU No. 2018-13 is effective for annual reporting periods, and interim periods within those years, beginning after December 15, 2019. The Company is currently in the process of evaluating the impact of the adoption of this ASU on its consolidated financial statements.
In November 2019, the FASB issued ASU No. 2019-08, Compensation—Stock Compensation (Topic 718) and Revenue from Contracts with Customers (Topic 606). The amendments in this Update require that an entity apply the guidance in Topic 718 to measure and classify share-based payment awards granted to a customer. The amount recorded as a reduction in the transaction price should be based on the grant-date fair value of the share-based payment award. The guidance in ASU No. 2019-08 is effective fiscal years beginning after December 15, 2019, and interim periods within fiscal years beginning after December 15, 2020. The Company is currently in the process of evaluating the impact of the adoption of this ASU on its consolidated financial statements.
| F-13 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Recently issued accounting pronouncements (continued)
In December 2019, the FASB Issued ASU No. 2019-12, Income Taxes (Topic 740) Simplifying the Accounting for Income Taxes. The amendments in this Update simplify the accounting for income taxes by removing certain exceptions to the general principles in Topic 740. The amendments also improve consistent application of and simplify U.S. GAAP for other areas of Topic 740 by clarifying and amending existing guidance. For public business entities, the amendments in this Update are effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020. The Company is currently in the process of evaluating the impact of the adoption of this ASU on its consolidated financial statements.
The Company does not believe that any other recently issued, but not yet effective accounting pronouncements, if adopted, would have a material effect on the consolidated financial statements.
Reclassifications
The Company has made certain reclassifications to conform its prior periods’ data to the current presentation, including the effects of the 200-for-1 reverse stock split that occurred on January 15, 2020. These reclassifications had no effect on the reported results of operations or cash flows.
NOTE 3 – INVENTORIES
Inventories consist of the following as of December 31:
| 2019 | 2018 | |||||||
| Finished goods | $ | 414,031 | $ | 96,750 | ||||
| Raw materials | 763,800 | 1,383,630 | ||||||
| Total inventories | $ | 1,177,831 | $ | 1,480,380 | ||||
As of December 31, 2019 and 2018, $763,830 and $1,383,630, respectively, in raw materials were held at the manufacturer’s facility for future production. Additionally, as of December 31, 2019 and 2018, $407,756 and $48,981, respectively, in finished goods were held at the manufacturer’s facility for shipment.
| F-14 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 4 – INTANGIBLE ASSETS, net
Intangible assets, net, consists of the following as of December 31:
| 2019 | 2018 | |||||||
| Patents | $ | 614,003 | $ | 578,326 | ||||
| Less accumulated amortization | (332,081 | ) | (292,512 | ) | ||||
| 281,922 | 285,814 | |||||||
| Patents pending | 138,451 | 148,720 | ||||||
| Total intangible assets, net | $ | 420,373 | $ | 434,534 | ||||
Patents are amortized straight-line over a period of fifteen years. Amortization expense was $39,569 and $28,669 for the years ended December 31, 2019 and 2018, respectively. During the year ended December 31, 2019, patent costs of $36,205 were expensed as a result of the abandonment of patents.
The Company has capitalized costs for several patents that are still pending. In those instances, the Company has not recorded any amortization. The Company will commence amortization when these patents are approved.
The Company has 29 issued patents, including 14 in the U.S. and 15 outside the U.S., and one patent pending outside the U.S that will expire between 2023 and 2028, subject to patent term extensions. The Company also has four additional patents pending that if issued would extend patent coverage in the U.S. and outside the U.S. to 2039-2041, one of which was filed subsequent to year-end.
NOTE 5 –ACCRUED SEPARATION COSTS
On August 9, 2016, the Company entered into a separation agreement with an employee to pay $118,635 of accrued compensation over nine-years. This amount is included in accrued payroll and payroll related expenses in the accompanying consolidated balance sheets. This amount does not yield interest and matures as follows for the years ended December 31:
| 2020 | $ | 9,000 | ||
| 2021 | 12,000 | |||
| 2022 | 12,000 | |||
| 2023 | 18,000 | |||
| 2024 | 18,000 | |||
| Thereafter | 23,635 | |||
| 92,635 | ||||
| Less current portion | (9,000 | ) | ||
| $ | 83,635 |
| F-15 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 6 – RELATED PARTY NOTES PAYABLE
Related party notes payable consisted of the following as of December 31:
| 2019 | 2018 | |||||||
| Inventory financing. On January 11, 2019, the Company entered into a $1,000,000 revolving inventory financing facility with a lender that is also a current stockholder that beneficially owns more than 5% of the Company’s common stock. Use of proceeds from this facility is limited to the purchase of inventory, including raw materials, intermediates, and finished goods, unless otherwise waived by the lender. This facility accrues interest at the rate of 12% per annum, is unsecured, and matures in three years from origination. This facility requires monthly interest payments. | $ | 1,000,000 | $ | - | ||||
| Officer loan. On June 26, 2019, the Company borrowed $75,000 from the Chief Executive Officer of the Company with principal and interest due on August 26, 2019, which was subsequently extended to June 30, 2020. This note accrues interest at the rate of 4.5% per annum and is unsecured. | 75,000 | - | ||||||
| Promissory note. On May 20, 2019, the Company entered into a $400,000 promissory note with a lender that is also a current stockholder that beneficially owns more than 5% of the Company’s common stock. On July 10, 2019, this note was amended to increase the principal sum by an additional $100,000. This note accrues interest at the rate of 12% per annum, is unsecured, and originally matured on August 20, 2019, which was subsequently extended to June 30, 2020. All principal and accrued interest is due on the maturity date. | 500,000 | - | ||||||
| Total notes payable | 1,575,000 | - | ||||||
| Less current portion | (575,000 | ) | - | |||||
| Long term notes payable | $ | 1,000,000 | $ | - | ||||
Interest expense
The Company incurred interest charges of $147,605 and $0 during the years ended December 31, 2019 and 2018, respectively, on these notes payable of which $47,413 was accrued and payable as of December 31, 2019.
| F-16 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 6 – RELATED PARTY NOTES PAYABLE (continued)
Maturities
Future maturities of notes payable are as follows as of December 31:
| 2020 | $ | 575,000 | ||
| 2021 | - | |||
| 2022 | 1,000,000 | |||
| $ | 1,575,000 |
NOTE 7 – RELATED PARTY CONVERTIBLE NOTES PAYABLE
Related party convertible notes payable consisted of the following as of December 31:
| 2019 | 2018 | |||||||
| Convertible note 2019-02. On July 19, 2019, the Company issued a convertible note payable in the amount $815,217, with an original issue discount of $65,217 in exchange for $750,000. This note accrues interest at 8% per annum and matures on June 30, 2020. This note and accrued interest may convert into shares of common stock at the conversion price then in effect (initially $24 per share, subject to adjustment, which resulted in a beneficial conversion feature) any time at the holder’s option or automatically upon a qualified financing of at least $5 million at the lower of the conversion price then in effect or a 25% discount to the offering price. The conversion price is subject to adjustment upon the issuance of the Company’s common stock or securities convertible into common stock at a price per share less than the then prevailing conversion price, other than specified exempt issuances; accordingly, on November 8, 2019, the conversion price was adjusted to $14 per share, which resulted in a beneficial conversion feature. (The conversion price was further adjusted after December 31, 2019 in connection with subsequent issuances and was equal to $4.27 per share as of March 30, 2020.) This note was also issued with a detachable warrant to purchase 7,500 shares of stock at $24 per share, which shall be adjusted in accordance with any adjustment to the conversion price of this note; accordingly, on November 8, 2019, the exercise price was adjusted to $14 per share. (The exercise price was further adjusted after December 31, 2019 in connection with subsequent issuances and was equal to $4.27 per share as of March 30, 2020.) The valuation of the conversion feature and detachable warrants and intrinsic value of the beneficial conversion feature resulted in the recognition of an additional $582,533 discount on this note. This note requires monthly interest payments. | $ | 815,217 | $ | - | ||||
| F-17 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 7 – RELATED PARTY CONVERTIBLE NOTES PAYABLE (continued)
| 2019 | 2018 | |||||||
| Convertible note 2019-07. On October 16, 2019, the Company issued a convertible note payable in the amount $217,391, with an original issue discount of $17,391 in exchange for $200,000. This note accrues interest at 8% per annum and matures on June 30, 2020. This note and accrued interest may convert into shares of common stock at the conversion price then in effect (initially $24 per share, subject to adjustment) any time at the holder’s option or automatically upon a qualified financing of at least $5 million at the lower of the conversion price then in effect or a 25% discount to the offering price. The conversion price is subject to adjustment upon the issuance of the Company’s common stock or securities convertible into common stock at a price per share less than the then prevailing conversion price, other than specified exempt issuances; accordingly, on November 8, 2019, the conversion price was adjusted to $14 per share, which resulted in a beneficial conversion feature. (The conversion price was further adjusted after December 31, 2019 in connection with subsequent issuances and was equal to $4.27 per share as of March 30, 2020.) This note was also issued with a detachable warrant to purchase 2,000 shares of stock at $24 per share, which shall be adjusted in accordance with any adjustment to the conversion price of this note; accordingly, on November 8, 2019, the exercise price was adjusted to $14 per share. (The exercise price was further adjusted after December 31, 2019 in connection with subsequent issuances and was equal to $4.27 per share as of March 30, 2020.) The valuation of the conversion feature and detachable warrants and intrinsic value of the beneficial conversion feature resulted in the recognition of an additional $110,783 discount on this note. This note requires monthly interest payments. | 217,391 | - | ||||||
| Officer convertible note. On November 15, 2019, the Company issued a convertible note payable in the amount $100,000. This note accrues interest at 14% per annum and matures on June 30, 2020. This note and accrued interest may convert into shares of common stock at the conversion price of $20 per share. This note requires monthly interest payments. | 100,000 | - | ||||||
| Total notes payable | 1,132,608 | - | ||||||
| Less original issue discounts | (82,608 | ) | - | |||||
| Related party convertible notes payable, net | 1,050,000 | - | ||||||
| Less conversion rights, warrant, and beneficial conversion feature discounts | (693,316 | ) | - | |||||
| Plus amortization of discounts | 295,037 | - | ||||||
| Total convertible notes payable, net | $ | 651,721 | $ | - | ||||
| F-18 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 7 – RELATED PARTY CONVERTIBLE NOTES PAYABLE (continued)
Discounts
Total discounts of $775,924 are amortized using the interest method, which resulted in amortization recorded as interest expense of $295,037 for the year ended December 31, 2019.
Interest expense
The Company incurred interest charges of $35,132 and $0 during the years ended December 31, 2019 and 2018, on these related party convertible notes payable of which $8,205 was accrued and payable as of December 31, 2019.
Maturities
Future maturities of notes payable are as follows as of December 31:
| 2020 | $ | 1,132,608 | ||
| $ | 1,132,608 |
NOTE 8 – CONVERTIBLE NOTES PAYABLE
Convertible notes payable consisted of the following as of December 31:
| 2019 | 2018 | |||||||
| Convertible note 2019-01. On April 18, 2019, the Company issued a convertible note payable in the amount $150,000. This note accrues interest at 10% per annum and originally matured on December 31, 2019, which was subsequently extended to March 31, 2020. This note and accrued interest may convert into shares of common stock at the conversion price then in effect (initially $24 per share, subject to adjustment, which resulted in a beneficial conversion feature) any time at the holder’s option or automatically upon maturity provided the 20-day volume weighted average price per share of the Company’s common stock upon maturity is at least $24 per share. The conversion price is subject to adjustment upon the issuance of the Company’s common stock or securities convertible into common stock at a price per share less than the then prevailing conversion price, other than specified exempt issuances; accordingly, on November 8, 2019, the conversion price was adjusted to $14 per share, which resulted in a beneficial conversion feature. (The conversion price was further adjusted after December 31, 2019 in connection with subsequent issuances and was equal to $4.27 per share as of March 30, 2020.) This note was also issued with a detachable warrant to purchase 2,500 shares of stock at $40 per share. The valuation of the conversion feature and detachable warrants and intrinsic value of the beneficial conversion feature resulted in the recognition of an additional $199,012 discount on this note. This note was fully repaid as of March 17, 2020. | $ | 150,000 | $ | - | ||||
| F-19 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 8 – CONVERTIBLE NOTES PAYABLE (continued)
| 2019 | 2018 | |||||||
| Convertible note 2019-03. On September 4, 2019, the Company issued a convertible note payable in the amount $108,696, with an original issue discount of $8,696 in exchange for $100,000. This note accrues interest at 8% per annum and matures on June 30, 2020. This note and accrued interest may convert into shares of common stock at $24 per share any time at the holder’s option, which resulted in a beneficial conversion feature. If this note, or any portion thereof, has not been repaid or converted in full on or prior to the maturity date, then repayment of the unpaid principal balance plus any accrued and unpaid interest thereon, shall be amortized over the following thirty-six (36) months. This note was also issued with a detachable warrant to purchase 1,000 shares of stock at $24 per share. The valuation of the detachable warrants resulted in the recognition of an additional $18,326 discount on this note. This note requires monthly interest payments. | 108,696 | - | ||||||
| Convertible note 2019-04. On September 25, 2019, the Company issued a convertible note payable in the amount $54,348, with an original issue discount of $4,348 in exchange for $50,000. This note accrues interest at 8% per annum and matures on June 30, 2020. This note and accrued interest may convert into shares of common stock at $24 per share any time at the holder’s option. If this note, or any portion thereof, has not been repaid or converted in full on or prior to the maturity date, then repayment of the unpaid principal balance plus any accrued and unpaid interest thereon, shall be amortized over the following thirty-six (36) months. This note was also issued with a detachable warrant to purchase 500 shares of stock at $24 per share. The valuation of the detachable warrants resulted in the recognition of an additional $4,190 discount on this note. This note requires monthly interest payments. | 54,348 | - | ||||||
| Convertible note 2019-05. On October 3, 2019, the Company issued a convertible note payable in the amount $27,174, with an original issue discount of $2,174 in exchange for $25,000. This note accrues interest at 8% per annum and matures on June 30, 2020. This note and accrued interest may convert into shares of common stock at $24 per share any time at the holder’s option. If this note, or any portion thereof, has not been repaid or converted in full on or prior to the maturity date, then repayment of the unpaid principal balance plus any accrued and unpaid interest thereon, shall be amortized over the following thirty-six (36) months. This note was also issued with a detachable warrant to purchase 250 shares of stock at $24 per share. The valuation of the detachable warrants resulted in the recognition of an additional $2,705 discount on this note. This note requires monthly interest payments. | 27,174 | - | ||||||
| F-20 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 8 – CONVERTIBLE NOTES PAYABLE (continued)
| 2019 | 2018 | |||||||
| Convertible note 2019-06. On October 10, 2019, the Company issued a convertible note payable in the amount $27,174, with an original issue discount of $2,174 in exchange for $25,000. This note accrues interest at 8% per annum and matures on June 30, 2020. This note and accrued interest may convert into shares of common stock at $24 per share any time at the holder’s option. If this note, or any portion thereof, has not been repaid or converted in full on or prior to the maturity date, then repayment of the unpaid principal balance plus any accrued and unpaid interest thereon, shall be amortized over the following thirty-six (36) months. This note was also issued with a detachable warrant to purchase 250 shares of stock at $24 per share. The valuation of the detachable warrants resulted in the recognition of an additional $2,505 discount on this note. This note requires monthly interest payments. | 27,174 | - | ||||||
| Convertible note 2019-08. On October 23, 2019, the Company issued a convertible note payable in the amount $108,696, with an original issue discount of $8,696 in exchange for $100,000. This note accrues interest at 8% per annum and matures on June 30, 2020. This note and accrued interest may convert into shares of common stock at $24 per share any time at the holder’s option. If this note, or any portion thereof, has not been repaid or converted in full on or prior to the maturity date, then repayment of the unpaid principal balance plus any accrued and unpaid interest thereon, shall be amortized over the following thirty-six (36) months. This note was also issued with detachable warrants to purchase 1,250 shares of stock at $30 per share and 1,250 shares of stock at $40 per share. The valuation of the detachable warrants resulted in the recognition of an additional $21,363 discount on this note. This note requires monthly interest payments. | 108,696 | - | ||||||
| Convertible note 2019-09. On October 29, 2019, the Company issued a convertible note payable in the amount $27,174, with an original issue discount of $2,174 in exchange for $25,000. This note accrues interest at 8% per annum and matures on June 30, 2020. This note and accrued interest may convert into shares of common stock at $24 per share any time at the holder’s option. If this note, or any portion thereof, has not been repaid or converted in full on or prior to the maturity date, then repayment of the unpaid principal balance plus any accrued and unpaid interest thereon, shall be amortized over the following thirty-six (36) months. This note was also issued with a detachable warrant to purchase 250 shares of stock at $24 per share. The valuation of the detachable warrants resulted in the recognition of an additional $2,295 discount on this note. This note requires monthly interest payments. |
27,174 |
- | ||||||
| F-21 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 8 – CONVERTIBLE NOTES PAYABLE (continued)
| 2019 | 2018 | |||||||
| Convertible note 2019-10. On November 8, 2019, the Company issued a convertible note payable in the amount $16,304, with an original issue discount of $1,304 in exchange for $15,000. This note accrues interest at 8% per annum and matures on June 30, 2020. This note and accrued interest may convert into shares of common stock at $14 per share any time at the holder’s option, which resulted in a beneficial conversion feature. If this note, or any portion thereof, has not been repaid or converted in full on or prior to the maturity date, then repayment of the unpaid principal balance plus any accrued and unpaid interest thereon, shall be amortized over the following thirty-six (36) months. This note was also issued with a detachable warrant to purchase 150 shares of stock at $14 per share. The valuation of the conversion feature and detachable warrants and intrinsic value of the beneficial conversion feature resulted in the recognition of an additional $3,279 discount on this note. This note requires monthly interest payments. |
16,304 |
- | ||||||
| Total notes payable | 519,566 | - | ||||||
| Less original issue discounts | (29,566 | ) | - | |||||
| Convertible notes payable, net | 490,000 | - | ||||||
| Less conversion rights, warrant, and beneficial conversion feature discounts | (253,675 | ) | - | |||||
| Plus amortization of discounts | 121,964 | - | ||||||
| Total convertible notes payable, net | $ | 358,289 | $ | - | ||||
Discounts
Total discounts of $283,241 are amortized using the interest method, which resulted in amortization recorded as interest expense of $121,964 for the year ended December 31, 2019, respectively.
Interest expense
The Company incurred interest charges of $17,877 and $0 during the years ended December 31, 2019 and 2018, respectively, on these notes payable of which $13,114 was accrued and payable as of December 31, 2019.
Maturities
Future maturities of notes payable are as follows as of December 31:
| 2020 | $ | 519,566 | ||
| $ | 519,566 |
| F-22 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 9 – DERIVATIVE FINANCIAL INSTRUMENTS
The Company has identified the embedded derivatives related to the convertible notes described in Notes 7 and 8. These embedded derivatives included certain conversion and reset features. The accounting treatment of derivative financial instruments requires that the Company record fair value of these derivative liabilities as of the inception date of those convertible notes and each subsequent reporting date.
The Company estimates the fair value of these derivative liabilities using the Black-Scholes valuation model. The initial value is used in the determination of a note discount with each subsequent change in fair value as a component of operations. The range of fair value assumptions used for derivative financial instruments during the year ended December 31, 2019, were as follows:
| Dividend yield | 0.0% | |
| Risk-free rate | 1.75% - 2.44% | |
| Volatility | 102% - 175% | |
| Expected term | 1 year |
Volatility was calculated based on the historical volatility of the Company. The risk-free interest rate used was based on the U.S. Treasury constant maturity rate in effect at the time of grant for the expected term of the derivative liabilities to be valued. The expected dividend yield was zero, because the Company does not anticipate paying a dividend within the relevant timeframe.
For the year ended December 31, 2019, the Company recognized total derivative liabilities and convertible note discounts of $471,000 based on the fair value at the convertible notes’ inception dates. These derivative liabilities were subsequently revalued at $827,314 as of December 31, 2019, which resulted in a loss of $356,314 on the change in value of these derivative liabilities.
The following table presents the three-level hierarchy prescribed by U.S. GAAP for derivative liabilities since it is a liability that is measured and recognized at fair value on a recurring basis as of:
| Level 1 | Level 2 | Level 3 | ||||||||||
| December 31, 2019 | - | - | $ | 827,314 | ||||||||
NOTE 10 – STOCKHOLDERS’ DEFICIT
Reverse Stock Split
On January 15, 2020, the Company effected a 200-for-1 reverse stock split (the “Reverse Stock Split”) of its issued and outstanding shares of common stock. The Reverse Stock Split did not change the number of shares of common stock authorized for issuance, the par value of the common stock, or any other terms of the common stock. No fractional shares were issued in the Reverse Stock Split and any remaining share fractions were rounded up to the next whole share. Under the terms and conditions of outstanding options, warrants, and other convertible securities, the number of underlying shares of common stock and the exercise prices or conversion prices thereof were proportionately adjusted for the Reverse Stock Split. All share and per share amounts reported in the consolidated financial statements reflect the Reverse Stock Split.
Self-directed stock issuance 2019
During the year ended December 31, 2019, the Company sold securities in a self-directed offering to existing stockholders of the Company in the aggregate amount of $245,000, respectively, at $60 per unit. Each $60 unit consisted of 2 shares of restricted common stock (8,169 shares) and a five-year warrant to purchase 1 share of restricted common stock (4,085 warrant shares) at $40 per share.
| F-23 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 10 – STOCKHOLDERS’ DEFICIT (continued)
Warrant exchange offering
In June 2018, the Company commenced an offering to exchange outstanding warrants for shares of common stock under a Form S-4 Registration Statement. These shares of common stock were issued to warrant holders in exchange for (i) their outstanding warrants to purchase shares of common stock at $125 per share, and (ii) cash payment of $30 per share. This offering closed on July 27, 2018, and resulted in an exchange of 48,033 warrants and $1,440,043 in gross proceeds for 48,033 shares of common stock. Stock issuance costs associated with this capital raise totaled $196,006, resulting in a net total of $1,244,037 raised in this offering.
Shares outstanding
As of December 31, 2019 and December 31, 2018, the Company had a total of 687,564 and 669,967, respectively, shares of common stock outstanding.
NOTE 11 – STOCK GRANTS
Director stock grants
During the years December 31, 2019 and 2018, the Company granted its independent directors an aggregate of 11,054 and 6,725, respectively, shares of restricted common stock in the Company. These shares were fully vested upon issuance. The increase in number of shares issued was due to the expansion of the Board of Directors by two members effective June 1, 2018. The expense recognized for these grants based on the grant date fair value was $350,000 and $287,500 for the years ended December 31, 2019 and 2018, respectively.
Consultant stock grants
On April 10, 2017, the Company granted a consultant 500 shares of restricted common stock valued at $46 per share. These shares were subject to a risk of forfeiture and vested quarterly in arrears commencing on April 1, 2017. The Company recognized $0 and $5,750 in stock-based compensation related to this grant during the years ended December 31, 2019 and 2018, respectively.
On August 8, 2017, the Company granted a consultant 500 shares of restricted common stock valued at $35 per share. These shares were subject to a risk of forfeiture and vested 25% upon grant and quarterly in arrears thereafter commencing on September 1, 2017. The Company recognized $0 and $4,375 in stock-based compensation related to this grant during the years ended December 31, 2019 and 2018, respectively.
On December 31, 2018, the Company granted consultants 563 shares of restricted common stock valued at $40 per share. These shares were fully vested upon issuance. The Company recognized $22,500 in stock-based compensation related to these grants during the year ended December 31, 2018.
On March 31, 2019, the Company granted consultants 187 shares of restricted common stock valued at $34 per share. On June 30, 2019, the Company granted consultants 188 shares of restricted common stock valued at $25 per share. On September 30, 2019, the Company granted consultants 187 shares of restricted common stock valued at $17.80 per share. On December 31, 2019, the Company granted consultants 188 shares of restricted common stock valued at $12 per share. These shares were fully vested upon issuance. The Company recognized $16,650 in stock-based compensation related to these grants during the year ended December 31, 2019.
| F-24 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 12 – STOCK OPTION PLANS
On February 7, 2014, the Company adopted the 2014 Equity Compensation Plan. Under this plan, the Company may issue options to purchase shares of common stock to employees, directors, advisors, and consultants. The aggregate number of shares reserved under this plan upon adoption was 152,101. On April 16, 2015, the majority stockholder of the Company approved an increase in the shares reserved under this plan by 75,000 shares. On December 4, 2018, the stockholders of the Company approved an increase in the shares reserved under this plan by an additional 25,000 shares and authorized the annual increase of the shares reserved under this plan on January 1st of each year, at the discretion of the Board of Directors, by up to such number of shares that is equal to four percent (4%) of the shares of common stock issued and outstanding as of December 31st of the previous calendar year. Accordingly, effective as of January 1, 2020, the shares reserved under this plan were increased by 27,000 shares. An aggregate of 279,101 shares of common stock are presently reserved for issuance under this plan.
Under the terms of the 2014 Equity Compensation Plan and the 2006 Stock Incentive Plan (collectively, the “Plans”), incentive stock options may be granted to employees at a price per share not less than 100% of the fair market value at date of grant. If the incentive stock option is granted to a 10% stockholder, then the purchase or exercise price per share shall not be less than 110% of the fair market value per share of common stock on the grant date. Non-statutory stock options and restricted stock may be granted to employees, directors, advisors, and consultants at a price per share, not less than 100% of the fair market value at date of grant. Options granted are exercisable, unless specified differently in the grant documents, over a default term of ten years from the date of grant and generally vest over a period of four years.
A summary of stock option activity is as follows:
| Options | Weighted average exercise price |
Weighted average remaining contractual term in years |
Aggregate intrinsic value |
|||||||||||||
| Outstanding January 1, 2018 | 191,119 | $ | 82.79 | 5.23 | $ | 562,579 | ||||||||||
| Exercisable January 1, 2018 | 181,119 | $ | 82.40 | 4.97 | $ | 562,579 | ||||||||||
| Canceled | (1,750 | ) | ||||||||||||||
| Granted | 14,168 | |||||||||||||||
| Exercised | (1,000 | ) | ||||||||||||||
| Forfeited | - | |||||||||||||||
| Outstanding December 31, 2018 | 202,537 | $ | 80.13 | 4.52 | $ | 987,064 | ||||||||||
| Exercisable December 31, 2018 | 185,837 | $ | 82.13 | 4.10 | $ | 967,064 | ||||||||||
| Canceled | (291 | ) | ||||||||||||||
| Granted | - | |||||||||||||||
| Exercised | - | |||||||||||||||
| Forfeited | - | |||||||||||||||
| Outstanding December 31, 2019 | 202,246 | $ | 80.14 | 3.52 | $ | - | ||||||||||
| Exercisable December 31, 2019 | 192,108 | $ | 81.32 | 3.26 | $ | - | ||||||||||
| F-25 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 12 – STOCK OPTION PLANS (continued)
The aggregate intrinsic value in the table above is before applicable income taxes and represents the excess amount over the exercise price option recipients would have received if all options had been exercised on December 31, 2019, based on a valuation of the Company’s stock for that day.
A summary of the Company’s non-vested options for the years ended December 31, 2019 and 2018 are presented below:
| Non-vested at January 1, 2018 | 10,000 | |||
| Granted | 14,168 | |||
| Vested | (5,718 | ) | ||
| Canceled | (1,750 | ) | ||
| Non-vested at December 31, 2018 | 16,700 | |||
| Granted | - | |||
| Vested | (6,271 | ) | ||
| Canceled | (291) | |||
| Non-vested at December 31, 2019 | 10,138 |
The Company estimates the fair value of stock options granted on each grant date using the Black-Scholes option valuation model and recognizes an expense ratably over the requisite service period. The range of fair value assumptions related to options issued were as follows for the years ended December 31:
| 2019 | 2018 |
|||||||
| Dividend yield | 0.0 | % | 0.0 | % | ||||
| Risk-free rate | 2.38% - 3.04 | % | 2.38% - 3.04 | % | ||||
| Volatility | 214% - 226 | % | 214% - 226 | % | ||||
| Expected term | 3 - 7 years | 3 - 7 years | ||||||
The expected volatility was calculated based on the historical volatility of the Company. The risk-free interest rate used was based on the U.S. Treasury constant maturity rate in effect at the time of grant for the expected term of the stock options to be valued. The expected dividend yield was zero, because the Company does not anticipate paying a dividend within the relevant timeframe. Due to a lack of historical information needed to estimate the Company’s expected term, it was estimated using the simplified method allowed.
The Company records forfeitures as they occur and reverses compensation cost previously recognized, in the period the award is forfeited, for an award that is forfeited before completion of the requisite service period.
| F-26 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 12 – STOCK OPTION PLANS (continued)
Stock option exercise
During the year ended December 31, 2018, the Company issued 786 shares of common stock in connection with the cashless exercise of stock options for 1,000 shares of common stock exercisable at $12 per share with 214 shares of common stock withheld with an aggregate fair market value equal to the aggregate exercise price.
Stock based compensation
The Company recognized stock-based compensation expense related to options during the years ended December 31:
| 2019 | 2018 | |||||||
| Service provider compensation | $ | 177,500 | $ | 124,896 | ||||
| Employee compensation | 164,438 | 205,250 | ||||||
| Total | $ | 341,938 | $ | 330,146 | ||||
NOTE 13 – WARRANTS
The following is a summary of the Company’s warrant activity:
| Warrants | Weighted average exercise price | Weighted average remaining contractual term in years | Aggregate intrinsic value | |||||||||||||
| Outstanding January 1, 2018 | 637,304 | $ | 47.13 | 3.15 | $ | 3,955,896 | ||||||||||
| Exercisable January 1, 2018 | 637,304 | $ | 47.13 | 3.15 | $ | 3,955,896 | ||||||||||
| Canceled | - | |||||||||||||||
| Granted | 1,579 | |||||||||||||||
| Exercised | (48,033 | ) | ||||||||||||||
| Expired | (510 | ) | ||||||||||||||
| Outstanding December 31, 2018 | 590,340 | $ | 40.65 | 2.32 | $ | 7,846,743 | ||||||||||
| Exercisable December 31, 2018 | 590,340 | $ | 40.65 | 2.32 | $ | 7,846,743 | ||||||||||
| Canceled | - | |||||||||||||||
| Granted | 20,985 | |||||||||||||||
| Exercised | - | |||||||||||||||
| Expired | (94,577 | ) | ||||||||||||||
| Outstanding December 31, 2019 | 516,748 | $ | 24.60 | 1.86 | $ | - | ||||||||||
| Exercisable December 31, 2019 | 516,748 | $ | 24.60 | 1.86 | $ | - | ||||||||||
| F-27 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 13 – WARRANTS (continued)
The Company estimates the fair value of warrants granted on each grant date using the Black-Scholes option valuation model. The expected volatility is calculated based on the historical volatility of the Company. The risk-free interest rate used is based on the U.S. Treasury constant maturity rate in effect at the time of grant for the expected term of the warrants to be valued. The expected dividend yield is zero, because the Company does not anticipate paying a dividend within the relevant timeframe. Due to a lack of historical information needed to estimate the Company’s expected term, it is estimated using the simplified method allowed.
The Company did not recognize any stock-based compensation expense related to warrants during the years ended December 31, 2019 and 2018, respectively.
Convertible note warrants
Warrants to purchase 16,900 shares of common stock at $14 to $40 per share were issued in connection with the issuance of convertible notes. These warrants were immediately vested and expire in five years and were recorded as discounts on the convertible notes in the aggregate amount of $125,545.
Warrant exchange offering
In June 2018, the Company commenced an offering to exchange outstanding warrants for shares of common stock under a Form S-4 Registration Statement. These shares of common stock were issued to warrant holders in exchange for (i) their outstanding warrants to purchase shares of common stock at $125 per share, and (ii) cash payment of $30 per share. This offering closed on July 27, 2018, and resulted in an exchange of 48,033 warrants and $1,440,043 in gross proceeds for 48,033 shares of common stock. Stock issuance costs associated with this capital raise totaled $196,006, resulting in a net total of $1,244,037 raised in this offering. As part of this offering, warrants to purchase 1,579 shares of common stock at $42 per share were issued to investment bankers for their services.
Warrant expiration
During the year ended December 31, 2019, warrants to purchase an aggregate of 94,577 shares of common stock expired. During the year ended December 31, 2018, warrants to purchase an aggregate of 510 shares of common stock expired.
| F-28 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 14 – INCOME TAXES
The following table presents a reconciliation of the statutory Federal rate and the Company’s effective tax rate for the years ended December 31:
| 2019 | 2018 | |||||||
| Change in valuation allowance | 12.80 | % | 17.69 | % | ||||
| Debt instruments | 3.19 | % | 0.00 | % | ||||
| Stock based compensation | 2.92 | % | 3.37 | % | ||||
| Accrued compensation | 1.03 | % | (0.28 | )% | ||||
| Interest expense | 0.85 | % | 0.00 | % | ||||
| Depreciation and amortization | 0.16 | % | 0.15 | % | ||||
| Other | 0.04 | % | 0.07 | % | ||||
| Tax provision (benefit) at Federal statutory rate | (21.00 | )% | (21.00 | )% | ||||
| Effective tax rate | 0.00 | % | 0.00 | % | ||||
The effective tax rate for the three and years ended December 31, 2019 and 2018, differs from the statutory rate of 21% for the years ended December 31, 2019 and 2018, respectively, as a result of state taxes (net of Federal benefit), permanent differences, and a reserve against deferred tax assets.
There was not a provision for income taxes for the years ended December 31, 2019 and 2018.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The following table presents significant components of the Company’s deferred tax assets and liabilities for the years ended December 31:
| 2019 | 2018 | |||||||
| DEFERRED TAX ASSETS, net: | ||||||||
| Net operating loss carryforwards | $ | 10,670,455 | $ | 9,633,893 | ||||
| Accrued compensation | 1,211,630 | 1,080,432 | ||||||
| Stock based compensation | 196,809 | 178,174 | ||||||
| Credit carryforwards | 58,122 | 52,592 | ||||||
| Interest expenses | 57,332 | - | ||||||
| Depreciation and amortization | (208,482 | ) | (63,917 | ) | ||||
| Debt instruments | (408,144 | ) | - | |||||
| Total | 11,577,722 | 10,881,174 | ||||||
| Less valuation allowance | (11,577,722 | ) | (10,881,174 | ) | ||||
| NET DEFERRED TAX ASSETS | $ | - | $ | - | ||||
As of December 31, 2019, the Company had a Federal net operating loss carryforward of $40,911,211. In addition, the Company had a net operating loss carryforward for Hawaii income tax purposes of $32,827,665 as of December 31, 2019. These amounts may be used to offset up to 80% of future taxable income and differ from the Company’s accumulated deficit due to permanent and temporary tax differences.
The Company’s valuation allowance was primarily related to the operating losses. The valuation allowance is determined in accordance with the provisions of ASC No. 740, Income Taxes, which requires an assessment of both negative and positive evidence when measuring the need for a valuation allowance. Based on the available objective evidence and the Company’s history of losses, management provides no assurance that the net deferred tax assets will be realized. As of December 31, 2019 and 2018, the Company has applied a valuation allowance against its deferred tax assets net of the expected income from the reversal of the deferred tax liabilities.
| F-29 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 14 – INCOME TAXES (continued)
Recent tax legislation
On December 22, 2017, the Tax Cuts and Jobs Act (“TCJA”) was enacted into law, which significantly changes existing U.S. tax law and includes numerous provisions that affect our business, such as reducing the U.S. federal statutory tax rate. The TCJA reduced the U.S. federal statutory tax rate from 35% to 21% effective January 1, 2018.
As a result of TCJA, the Company recorded a change in its deferred tax asset of approximately, $3.8 million for the year ended December 31, 2017, which was offset by an adjustment to the allowance.
NOTE 15 – BASIC AND DILUTED NET LOSS PER SHARE
The following table sets forth the computation of the Company’s basic and diluted net loss per share for the years ended December 31:
| 2019 | ||||||||||||
| Net
Loss (Numerator) |
Shares
(Denominator) |
Per
share amount |
||||||||||
| Basic loss per share | $ | (5,093,037 | ) | 680,152 | $ | (7.49 | ) | |||||
| Effect of dilutive securities—Common stock options and warrants | - | - | - | |||||||||
| Diluted loss per share | $ | (5,106,514 | ) | 680,152 | $ | (7.49 | ) | |||||
| 2018 | ||||||||||||
| Net
Loss (Numerator) |
Shares
(Denominator) |
Per
share amount |
||||||||||
| Basic loss per share | $ | (4,024,222 | ) | 637,028 | $ | (6.32 | ) | |||||
| Effect of dilutive securities—Common stock options and warrants | - | - | - | |||||||||
| Diluted loss per share | $ | (4,024,222 | ) | 637,028 | $ | (6.32 | ) | |||||
The following outstanding shares of common stock equivalents were excluded from the computation of diluted net loss per share for the periods presented because including them would have been antidilutive for the years ended December 31:
| 2019 | 2018 | |||||||
| Common stock underlying options | 202,246 | 202,537 | ||||||
| Common stock underlying warrants | 516,748 | 590,340 | ||||||
| Common stock underlying convertible notes | 105,360 | - | ||||||
| Total common stock equivalents | 824,354 | 792,877 | ||||||
| F-30 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 16 – LEASES
Manoa Innovation Center
The Company entered into an automatically renewable month-to-month lease for office space on August 13, 2010. Under the terms of this lease, the Company must provide a written notice 45 days prior to vacating the premises. Total rent expense under this agreement as amended was $37,915 and 39,302, for the years ended December 31, 2019 and 2018, respectively.
Fleet Lease
In January 2018, the Company entered into a vehicle lease arrangement with a rental company for three vehicles. The terms of the leases require monthly payments of $1,619 for three years. These leases convert to month-to-month leases in January 2021 unless terminated. The Company terminated one lease in August of 2019, which reduced the monthly payments to $1,002. Total lease expense under this agreement was $16,174 and $21,196 for the years ended December 31, 2019 and 2018, respectively.
Right-to-use leased asset and liability
As a result of the adoption of ASU No. 2016-02, Leases, on January 1, 2019, the Company recognized a right-to-use leased asset and liability for the Fleet Leases. The balance of this right-to-use asset and liability was $12,488 as of December 31, 2019.
Future minimum lease payments
Future minimum lease payments are as follows for the years ended December 31:
| 2020 | $ | 12,024 | ||
| 2021 | 1,002 | |||
| $ | 13,026 |
| F-31 |
Cardax, Inc., and Subsidiary
NOTES TO THE CONSOLIDATED
FINANCIAL STATEMENTS (continued)
NOTE 17 – COMMITMENTS
Patent payable
As part of the formation of the Company, a patent license was transferred to the Company. The original license began in 2006. Under the terms of the license the Company agreed to pay $10,000 per year through 2015 and royalties of 2% on any revenues resulting from the license. There were no revenues generated by this license during the years ended December 31, 2019 and 2018. The remaining obligation of $20,000 as of December 31, 2019 and 2018, is recorded as a part of accounts payable on the consolidated balance sheets. The license expired in February 2016.
Employee settlement
As of December 31, 2019 and 2018, the Company owed a former employee a severance settlement payable in the amount of $50,000 for accrued vacation benefits. As part of the severance settlement, a stock option previously granted to the former employee was fully vested and extended.
NOTE 18 – SUBSEQUENT EVENTS
The Company evaluated all material events through the date the financials were ready for issuance and noted the following non-recognized events for disclosure.
Potential Impact of COVID-19
In December 2019, a novel coronavirus was reported in China and the resulting COVID-19 has become a global pandemic as of the date of this report. This matter may negatively impact our results of operations; however, the related financial impact and duration cannot be reasonably estimated at this time.
Convertible Promissory Notes
The Company entered into convertible notes payable with lenders as set forth in the table below. Each of these notes and accrued interest thereon may convert into shares of our common stock at the conversion price set forth in the table below. Certain of these notes were issued with detachable five-year warrants to purchase shares of our common stock as set forth in the table below.
| Issuance Date | Principal Amount | Original Issue Discount | Gross Proceeds | Interest Rate | Maturity Date | Note Conversion Price Per Share | Number of Shares Underlying Warrants | Warrant Exercise Price Per Share | ||||||||||||||||||||||||
| January 6, 2020 | $ | 10,870 | $ | 870 | $ | 10,000 | 8% | (1) | June 30, 2020 | (4,5) | $ | 10.00 | (7) | 100 | $ | 10.00 | ||||||||||||||||
| January 21, 2020 | 262,500 | 12,500 | 250,000 | 10% | (2,3) | June 30, 2020 | (6) | 4.27 | (7) | - | (10,11) | - | ||||||||||||||||||||
| February 25, 2020 | 52,632 | 2,632 | 50,000 | 8% | (1) | June 30, 2020 | (4,5) | 7.50 | (8) | 500 | 7.50 | |||||||||||||||||||||
| March 16, 2020 | 250,000 | 20,000 | 230,000 | 10% | (2) | September 16, 2020 | (4) | 4.50 | (7,9) | - | (10,12) | 5.75 | ||||||||||||||||||||
| March 16, 2020 | 250,000 | 20,000 | 230,000 | 10% | (2) | September 16, 2020 | (4) | 4.50 | (7,9) | - | (10,12) | 5.75 | ||||||||||||||||||||
| Total | $ | 826,002 | $ | 56,002 | $ | 770,000 | 8-10% | 2020 | $ | 4.27-10.00 | 600 | $ | 5.75-10.00 | |||||||||||||||||||
| (1) | Accrued interest on this note is payable monthly in cash. | |
| (2) | Accrued interest on this note is payable upon maturity. | |
| (3) | One-time fixed interest charge equal to ten percent (10%). | |
| (4) | Prepayment of this note is not subject to a prepayment penalty or premium. | |
| (5) | If this note, or any portion thereof, has not been repaid or converted in full on or prior to the maturity date, then repayment of the unpaid principal balance plus any accrued and unpaid interest thereon, shall be amortized over the following thirty-six (36) months. | |
| (6) | Prepayment of this note is subject to a prepayment penalty or premium of fifteen percent (15%) for prepayments on or prior to March 31, 2020, twenty-five percent (25%) for prepayments on or prior to May 15, 2020, or thirty percent (30%) prior to June 30, 2020 | |
| (7) | This note and accrued interest thereon may convert into shares of our common stock any time at the holder’s option. | |
| (8) | This note and accrued interest thereon may convert into shares of our common stock any time at the holder’s option or automatically upon a qualified financing of at least $5 million at the lower of the conversion price then in effect or a twenty-five percent (25%) discount to the offering price. | |
| (9) | The conversion price of this note is subject to adjustment upon the issuance of our common stock or securities convertible into our common stock at a price per share less than the then prevailing conversion price, other than specified exempt issuances. | |
| (10) | No warrant was issued in connection with this note. | |
| (11) | 5,855 shares of our common stock were issued in connection with the purchase of this note. | |
| (12) | 5,000 shares of our common stock were issued as a commitment fee in connection with the purchase of this note. In addition, 27,777 shares of our common stock were issued in connection with the purchase of this note; provided, however, such shares must be returned to us if this note is fully repaid within six (6) months following the issuance date. |
On January 16, 2020, the $150,000 convertible note issued April 18, 2019 was amended as follows: (i) the maturity date was extended, as of December 31, 2019, to March 31, 2020; (ii) we agreed to make payments of accrued interest and principal, together with a prepayment penalty equal to twenty percent (20%) of the principal payment, in an aggregate amount of not less than $15,000 per month beginning February 1, 2020 until this note was repaid in full; and (iii) the lender agreed that we could incur additional indebtedness. On March 17, 2020, we fully repaid the remaining principal and interest due under this note, together with a reduced prepayment penalty equal to approximately ten percent (10%) of the principal payment, for an aggregate payment amount of $150,000, pursuant to an agreement between the holder and us, under which we also agreed to adjust the exercise price of the warrant issued in connection with this note to $6.25 per share.
***
| F-32 |